|
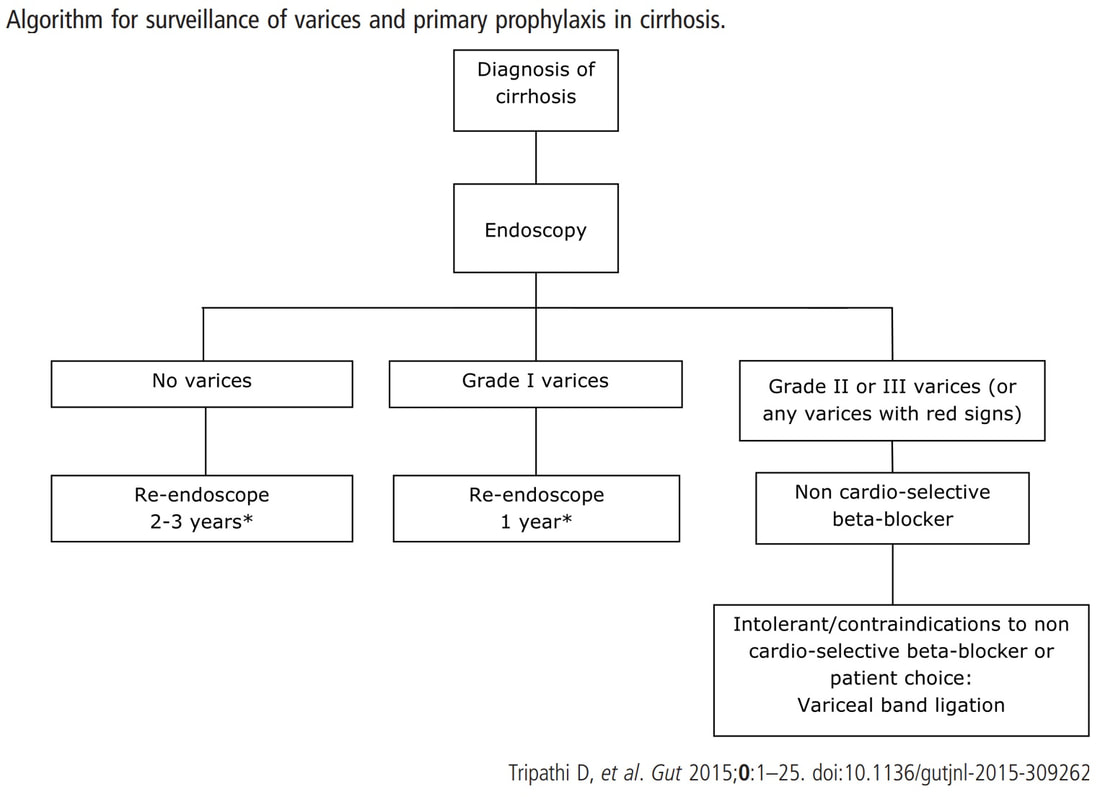
This patient is on a surveillance programme due to alcoholic liver disease but has never had a bleed. He is maintained on a non-cardioselective β blocker. WHEN WOULD YOU RECOMMEND THE NEXT SURVEILLANCE EXAMINATION?
■ None, this patient should be offered banding
Correct, if you decide that there are 'red signs' or the varices are grade II or III
■ In 12 months time
Correct, if you decide that there are no 'red signs' and that the varices are grade I
■ In 2-3 years time
Would only be correct if there are no varices at all!
explanation
I must admit that I don't like the current surveillance guidelines for patients with portal hypertension. This is a good example why! Although the LFT's had remained stable and the patient had remained abstinent, you could argue that there are 'red signs'. Of course, the presence of 'red signs' predicts progression of varices [J Hepatol 2003;38:266–72] and because the patient is already on a β-blocker, band ligation should now be started. The truth is that 'red signs' are common and have a poor agreement value between endoscopists.
If you decide that there are no 'red signs', and that the varices are grade I only (which depends on the degree of inflation of the oesophagus), the recommendation is to offer surveillance in 1 year. Finally, if you decide that the varices are now grade II (or III), variceal band ligation would be the logical next step. Thus, you can make an subjective argument for any of the above three treatment options. Of course, what you decide will be judged in hindsight. If you decide that these are not red signs and the patient re-presents with a bleed in 6 months time, you could be open to criticism for missing signs of progressive liver disease. Surely, in the modern era of FibroScans, it's possible to predict progression of portal hypertension non-invasively!!! Five years ago, the BAVENO VI workshop only mentioned in passing, that surveillance endoscopies may be avoided in patients with elastography values <20 kPa and a platelet counts >150,000 as these patients are at low risk of progression. Similarly increasing size of the spleen is another warning sign and could be looked for when these patients attend for screening for HCC's. The American guidelines suggest that there is no need to offer patients with untreated viral cirrhosis a screening endoscopy to search for varices IF elastography is <20 kPa and the platelets are >150. They concede that annual elastography and platelet counts may be less predictive in other causes of cirrhosis. However, the American guidelines advice continued surveillance if varices have been found in the past, particularly if liver injury is ongoing. The next Baveno conference in October 2021 will hopefully recommend non-invasive monitoring rather than endoscopy. It would be cheaper, less arduous for patients and offer less subjective findings! The now rather dated BSG guidelines are summarised in the graph below.
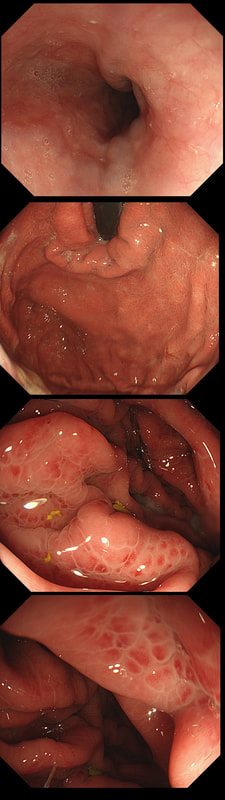
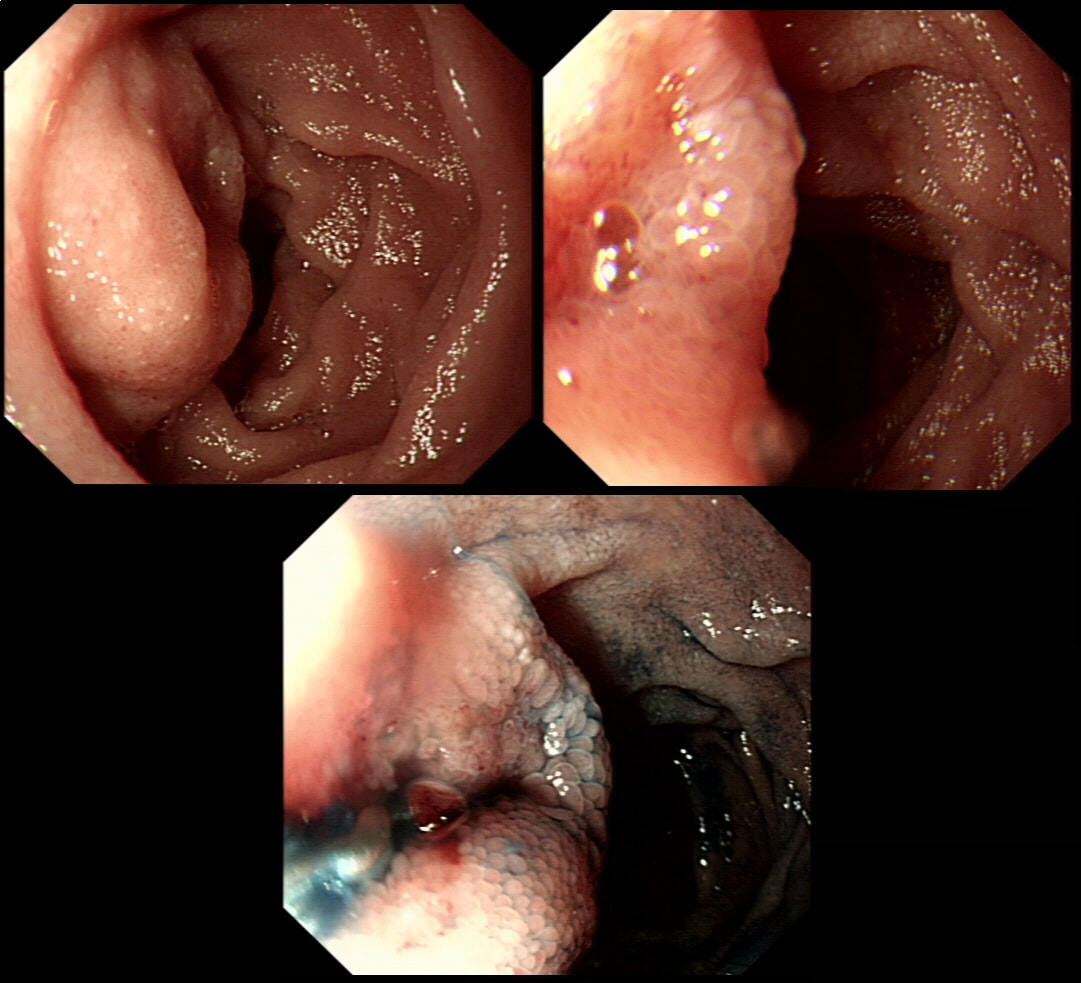
This was found at gastroscopy in a middle aged patient with anaemia
WHICH STATEMENT IS CORRECT?
■ The severity is related to the severity of the underlying disease
This is generally thought to be the case!
■ Its generally more marked in the distal vs the proximal stomach
It's the other way around actually
■ Beta blockers don't help
Beta blockers do reduce gastric perfusion
■ The condition is irreversible
A reduction in portal pressure improves the condition
explanation
Congestion secondary to portal hypertension is thought to be the primary cause of Portal Hypertensive Gastropathy. However, other factors may also play a part such as mucosal protective mechanisms, inflammatory response, local vascular tone, hepatic function, gastric mucosal perfusion, endotoxin, and gastric sucrose permeability, have been suggested to influence the development of Portal Hypertensive Gastropathy. However, because Portal Hypertensive Gastropathy is a dynamic condition which improves after liver transplantation or after TIPS, suggests that it IS portal hypertension which is the primary driver.
There is a correlation between Portal Hypertensive Gastropathy and Child-Pugh stage, HVPG (hepatic vein pressure gradient), MELD score, albumin level, bilirubin, platelet count, INR and even survival [Bang CS. BMC Gastro 2016;16(93] You are right, there is also a small oesophageal varix visible!
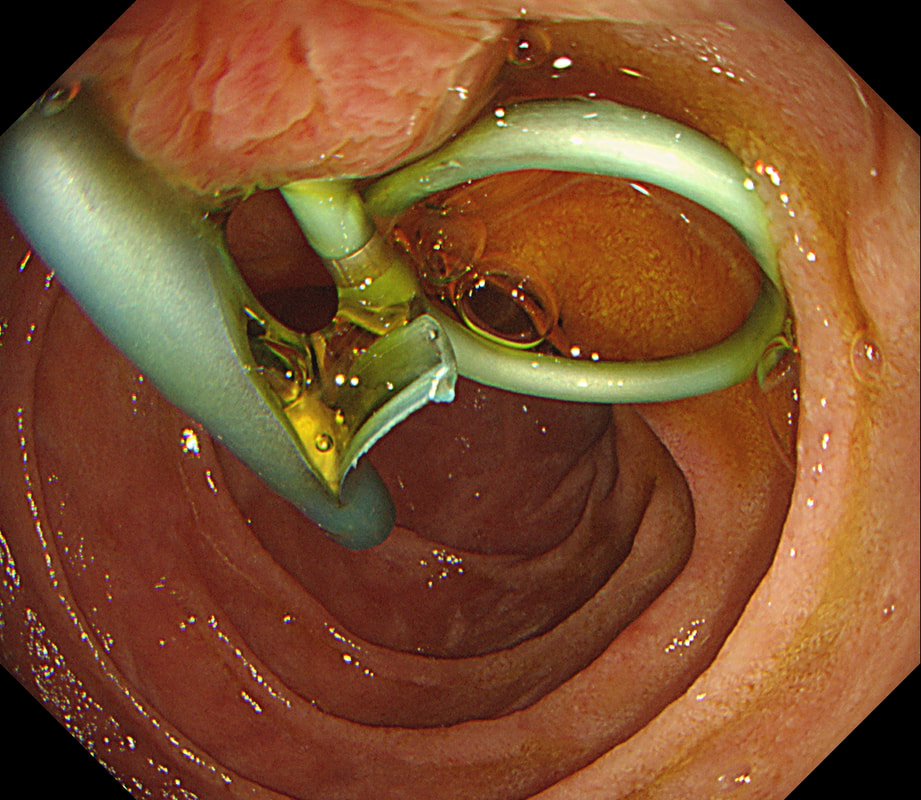
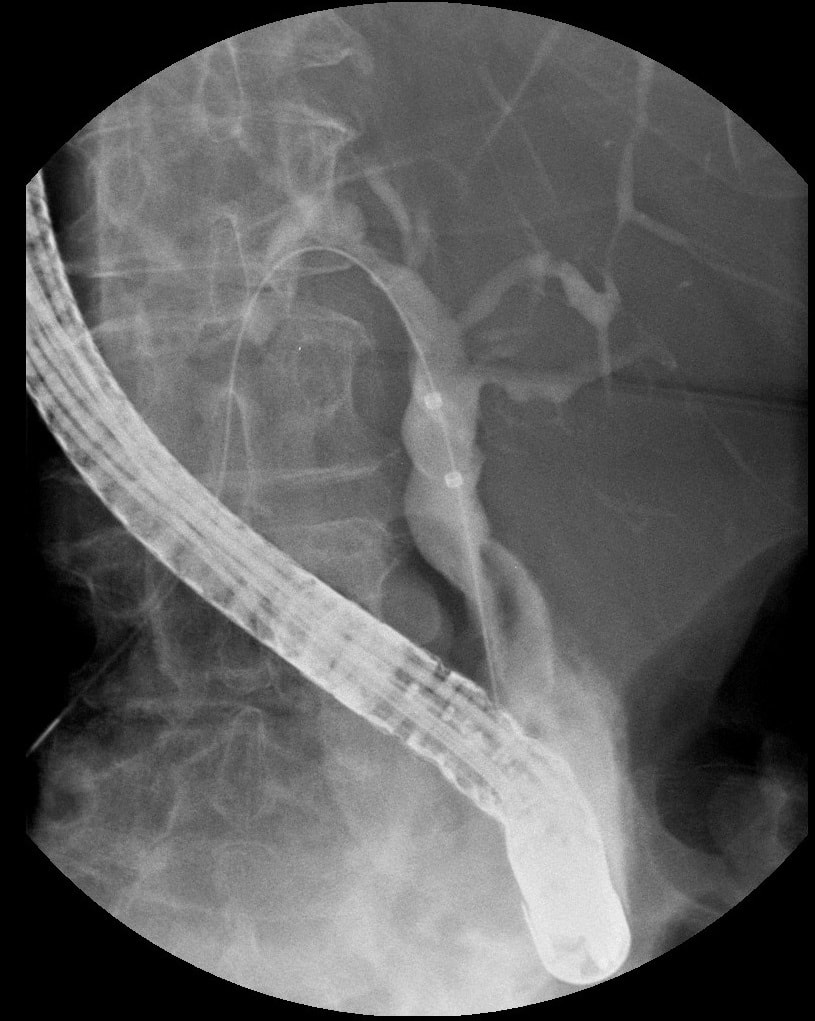
Patient has turned up to have his 'green' PD stent removed but I'm astounded to find two stents poking out of the papilla and they are both green !?
WHAT SHOULD I DO?
■ Remove the stent with a twist in it
Turned out to be correct!
■ Remove both stents
A dangerous attitude?
explanation
After scrutinising the X-rays, I concluded that the Pancreatic Duct stent was the 'single pig-tailed' stent (the one with the twist). Furthermore, the other one was draining bile. Obviously, the reason for the pig-tail is to stop the stent from migrating up the duct. PD stents are more likely to migrate than CBD stents and therefore the pig-tailed stent was more likely to be the PD stent even before I confirmed this on the images taken at the time of ERCP.
Well, this is one of the reasons why I don't like having stent removals on my list. Shouldn't it be the job of the person who placed to stent to also remove them?!
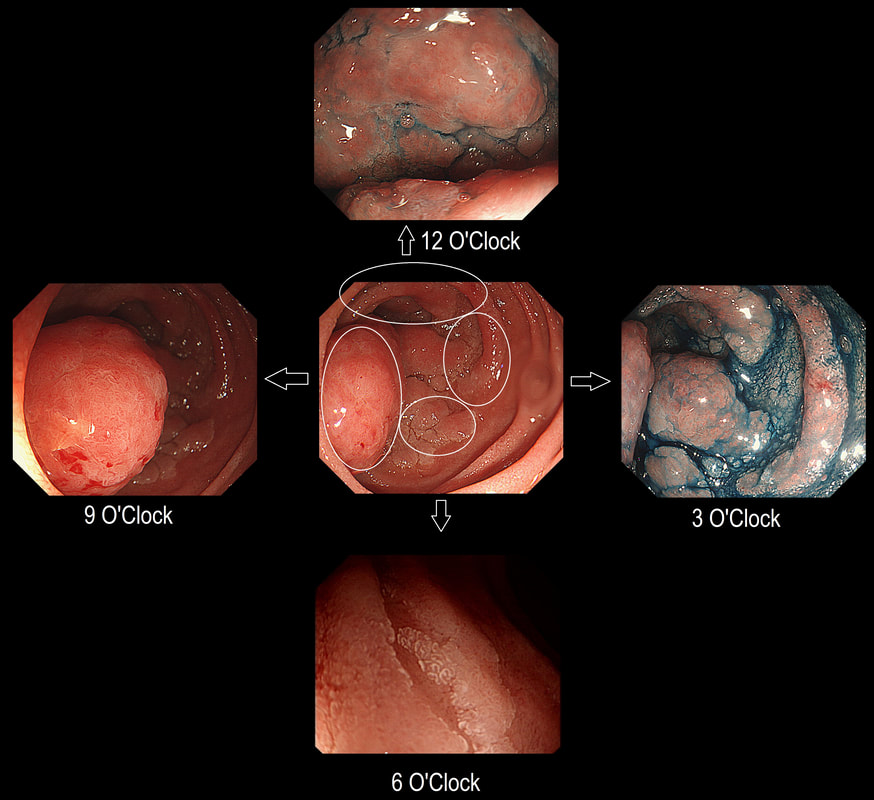
Here is a circumferential duodenal polyp situated in the second part of the duodenum. The patient is somewhat uncomfortable and your 'window of opportunity' is closing.
WHERE SHOULD YOU TARGET YOUR BIOPSY?
■ 12 O'clock
Looks amourphous and worrying but not the right answer
■ 3 O'clock
Wouldn't be my priority site
■ 6 O'clock
This is the least worrying spot!
■ 9 O'clock
Absolutely - for two reasons!
explanation
In general ampullary or peri-ampullary adenomas are far more likely to turn malignant than adenomas situated elsewhere in the duodenum. This is the first reason, why you should target your biopsy at 9 O'clock. There is a second reason and that is that the most 'chunky' polyp is at 9 O'clock. In my experience, the larger the 'polyp volume', the greater is the risk of malignant conversion.
So should we offer an ampullectomy? I would offer this to anyone with a life-expectancy of 10 years or more, I would consider (no promises) anyone with a life-expectancy of 5 years or more IF there is HGD on biopsies and there is no evidence of ductal obstruction (pt never had jaundice and there is no ductal dilatation or MRI or on EUS). Of course, an ampullectomy (probably better called a 'papillectomy') is dangerous but less dangerous than surgery. In Leeds we quote the following risks of a papillectomy: 1:10 risk of acute pancreatitis for up to a few months after the procedure, 1:10 risk of late bleeding for up to 2 weeks after the procedure, 1:50 risk of a perforation, a 1:10 risk of late papillary stenosis, 1:20 risk of acute cholangitis and a 1:200 risk of death Tiger country!!!
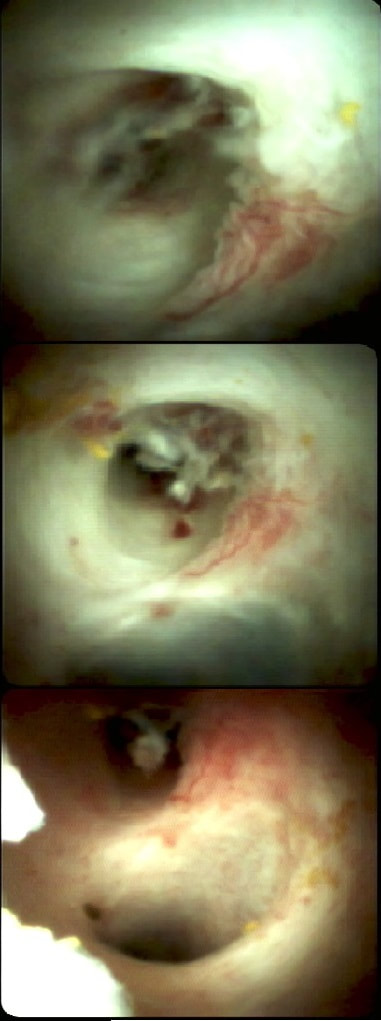
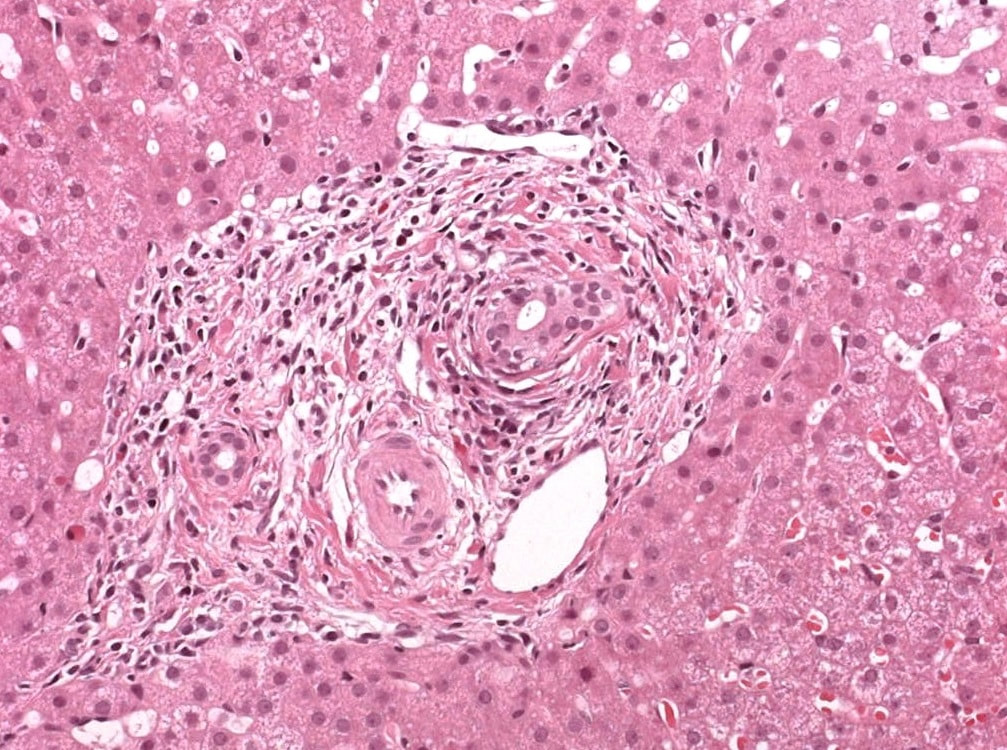
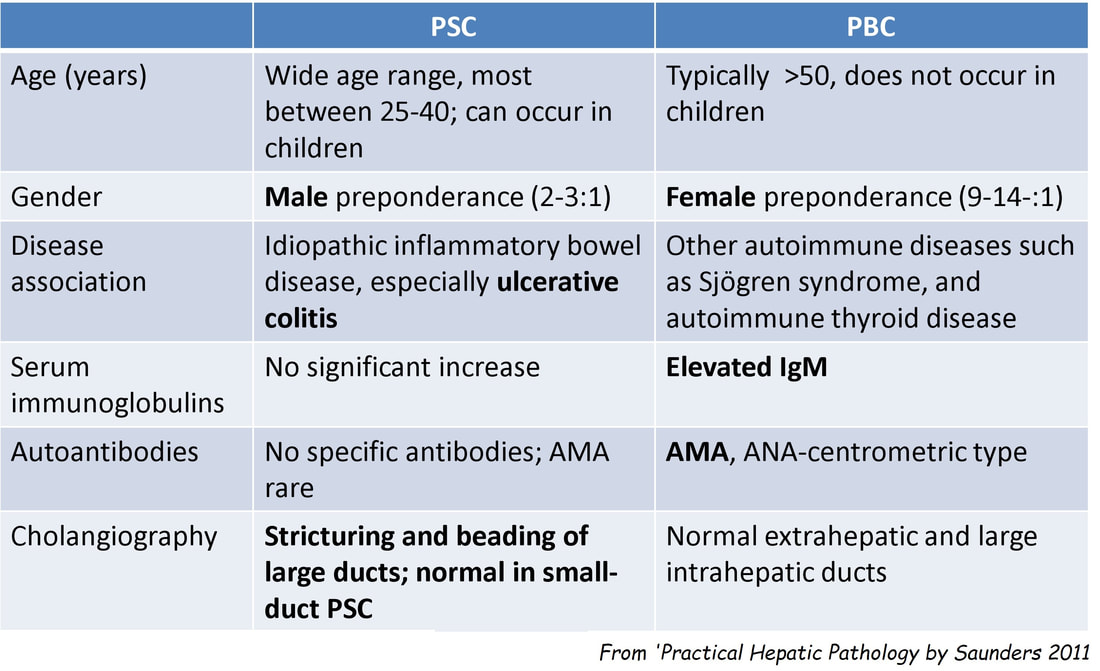
A full house of cholangiogram, cholangioscopy and histology of a 35 year old man with jaundice!
WHAT IS THE DIAGNOSIS?
■ Autoimmune hepatitis
But this should be diagnosed by blood tests?!
■ Alcoholic hepatitis
The last thing a patient would need is an ERCP
■ Primary Billiary Cirrhosis
But it's a man!
■ Primary Sclerosing cholangitis
Got it!
explanation
This is of course something of a curveball. Why would a patient with any of these causes of jaundice undergo an ERCP? Surely a clinical assessment, blood tests and an MRI would be the investigation of choice? Without any risk of giving the patient cholangitis!? Actually, the three images (cholangiogram, cholangioscopy and histology) are from three historical cases ☺!
MRI has a sensitivity of about 85% and specificity of around 95% for PSC. In most cases of PSC (87%), both intra and extra-hepatic bile ducts are involved , intrahepatic ducts alone in 10-25% and very rarely only the extrahepatic ducts (2%). Cholangioscopy shows patches of inflammation on the wall of the CBD and the cholangiogram did suggest some 'pruning' of the intra-hepatic biliary tree. A subsequent liver biopsy (we really did go all out), shows the typical peri-portal 'onion skin' inflammatory changes in keeping with PSC (although histology can't usually completely rule out other causes of biliary obstruction).
This 25 year old man presented with an attack of jaundice. Here are photographs of his duodenal ampulla and EUS
WHAT IS THE DIAGNOSIS?
■ Impacted gallstone
There is more than just a bulging ampulla
■ Pancreas divisum
Nope!
■ Mass in the head of pancreas
But 'mass' seems to be extending below ampulla
■ Choledochal cyst
Of course, you do ERCP's clearly!
■ Duodenal varices
Looks a little vascular and would be soft to touch!
explanation
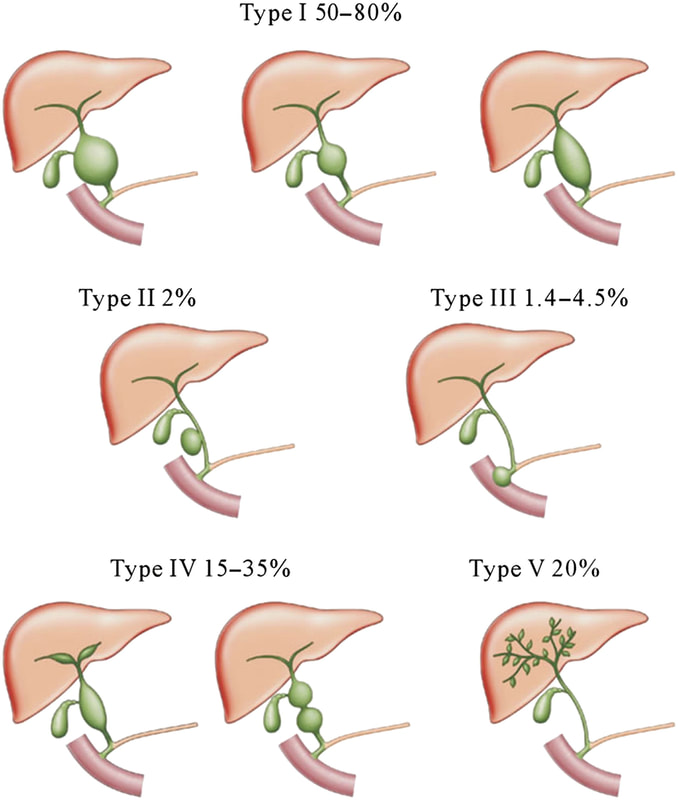
That EUS does confirm a cystic dilatation of the distal part of the common bile duct. Of course, the patient has a 'choledochal cyst' ! These are congenital cystic dilatations of parts of the biliary tree. In this case, only the distal CBD was involved, making this a 'type III' choledochal cyst which is located at the ampulla (see figure below).
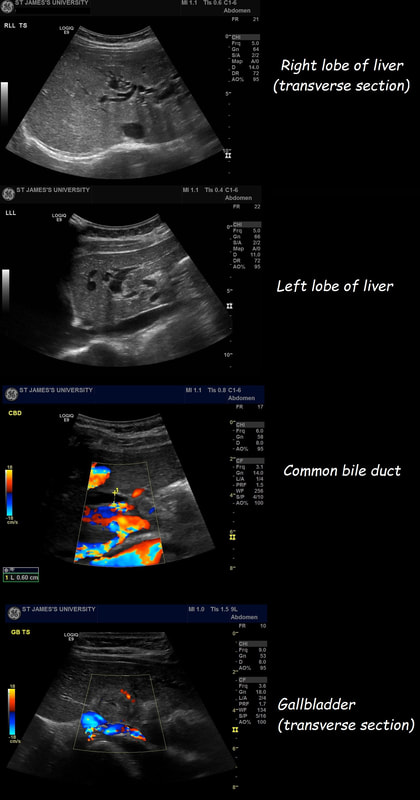
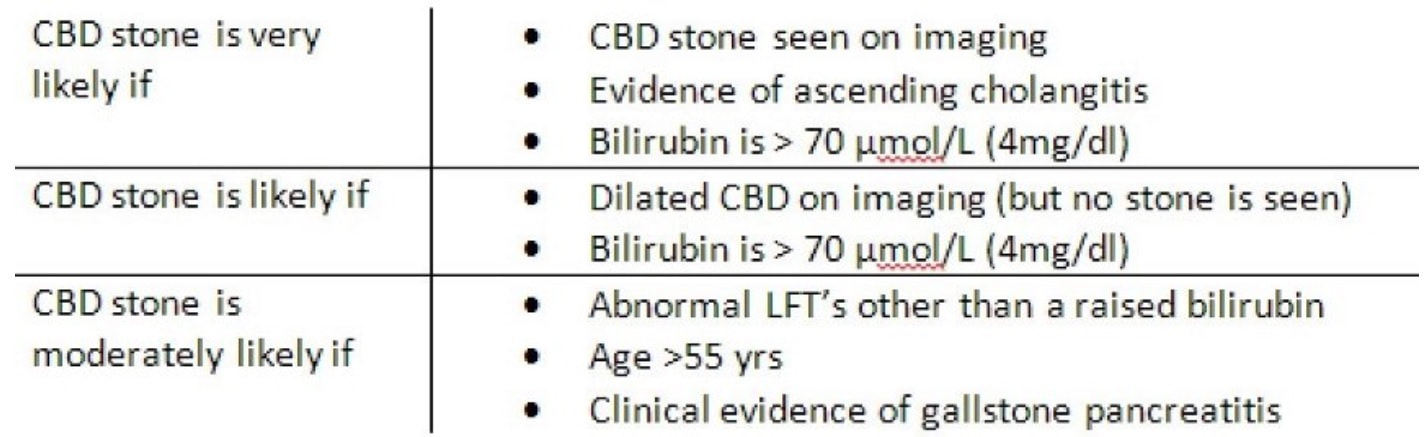
Most cases presents as jaundice or pain in infants. Chronic cholestasis may present later with biliary cirrhosis or cholangiocarcinoma. Fortunately, this case was discovered before cirrhosis and the patient was treated with surgery. A middle aged patient presents with obstructive jaundice. There is a history of upper abdominal pain for 2 months and jaundice for about 4 weeks. She has also lost some weight loss and had night sweats. On examination she is apyrexial, obviously jaundiced and slightly tender in the epigastrium Hb 98 MCV 71 WCC 4.17 Plat 338 CRP <5 (<10) Amylase 102 (<110) Bili 75 µmol/L ALT 910 iu/L ALP 302 iu/L Albumin 37 g/L INR 1.0 An abdominal ultrasound is carried out (images below) If you work outside of the UK, and actually do abdominal ultrasound examinations you will be able to see that the gallbladder is extremely thick walled and hyperaemic. There is also intrahepatic biliary duct dilatation but the CBD is of normal calibre (6mm) and no stones can be seen. I haven't included the images showing that the pancreas, pancreatic duct, liver, abdominal aorta, spleen and kidneys were all normal. WHAT IS YOUR CLINICAL DIAGNOSIS?
■ Biliary colic
There is no pain!
■ Acute cholecystitis
How then do you explain the jaundice?
■ Choledocholithiasis
Would explain the jaundice and a CBD stone can be missed on US
■ Ascending Cholangitis
But there is no fever, raised WCC or CRP !!!
■ Acalculous cholecystitis
Sure no stones seen in the gallbladder but why the jaundice?
explanation of the case (so far - there is more to follow!!! )
Choledocholithiasis should be your clinical diagnosis at this point. This is because the ‘strong likelihood criteria’ are fulfilled as follows;
To search further for gallstones, a CT scan was then requested (below). You can probably tell from the CT that The gallbladder is confirmed as thickened with some extrinsic compression of the bile duct at the porta hepatis to explain the intrahepatic ductal dilatation already seen on ultrasound. The distal bile duct is confirmed as collapsed with normal appearance of the pancreas, kidneys, adrenal glands and spleen. WHAT IS NOW THE DIAGNOSIS?
■ Mirizzi’s syndrome
Absolutely
■ Choledocholithiasis
Nope, no stones seen within the CBD
second explanatation (and yes there are further developments around the corner)
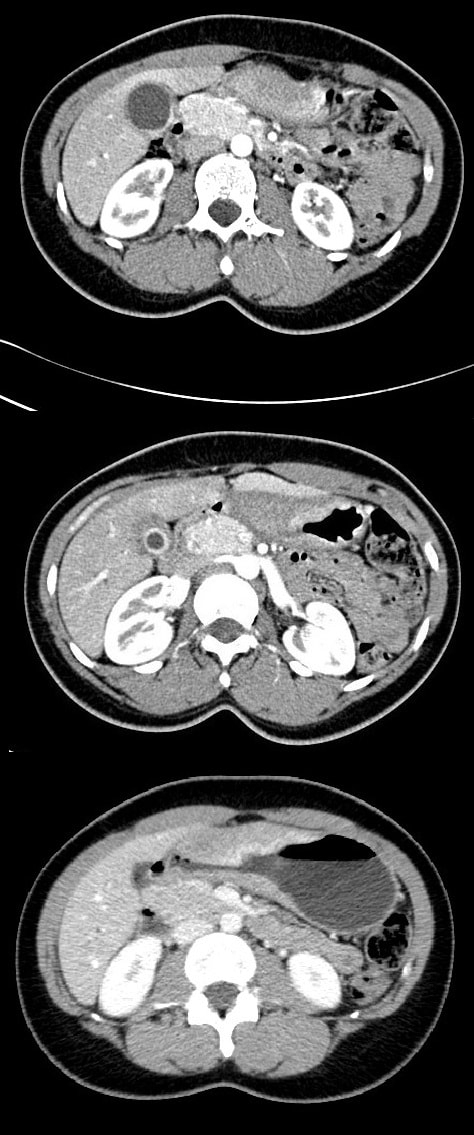
The CT report essentially describes a Mirizzi syndrome type 1. Pablo Mirizzi was an Argentinian Surgeon who first described the obstruction of the common hepatic duct (CHD) by an impacted stone in the cystic duct or Hartmann's pouch of the gallbladder. In Mirizzi syndrome type I there is no fistula between the gallbladder and CHD whilst type II-IV have a fistulous communication. Of course its difficult to tell on imaging if there is a fistula. For this reason the subtype of the Mirizzi syndrome is usually something discovered at surgery.
The patient does undergo a laparoscopic cholecystectomy but to the surgeons surprise there are no stones found within the thickened gallbladder or within the bile ducts. The gallbladder is analysed and the pathologists report that the: “Gallbladder measures 52 x 30 x 20 mm. The serosa is congested and wall thickness is 3 mm. No stone and no focal lesions. There is subacute cholecystitis with myofibroblastic proliferation of the wall and mild acute on chronic inflammation” Strangely enough, the jaundice does not resolve after surgery and for this reason an MRI scan is carried out which confirms a stricture at the common hepatic duct with mild intrahepatic ductal dilatation. At this point we decide to carry out an ERCP to sample the stricture and place a stent. Now you have all the pieces in this jigsaw and should know what's going on! WHAT DID WE MISS?
■ Primary cancer of the gallbladder
No cancer found in resection specimen!
■ Cholangiocarcinoma
You missed something on the video!
■ Gastric cancer
Well done, you spotted this on the video?
■ Duodenal cancer
But the duodenum look fine on video?!
FINAL EXPLANATION
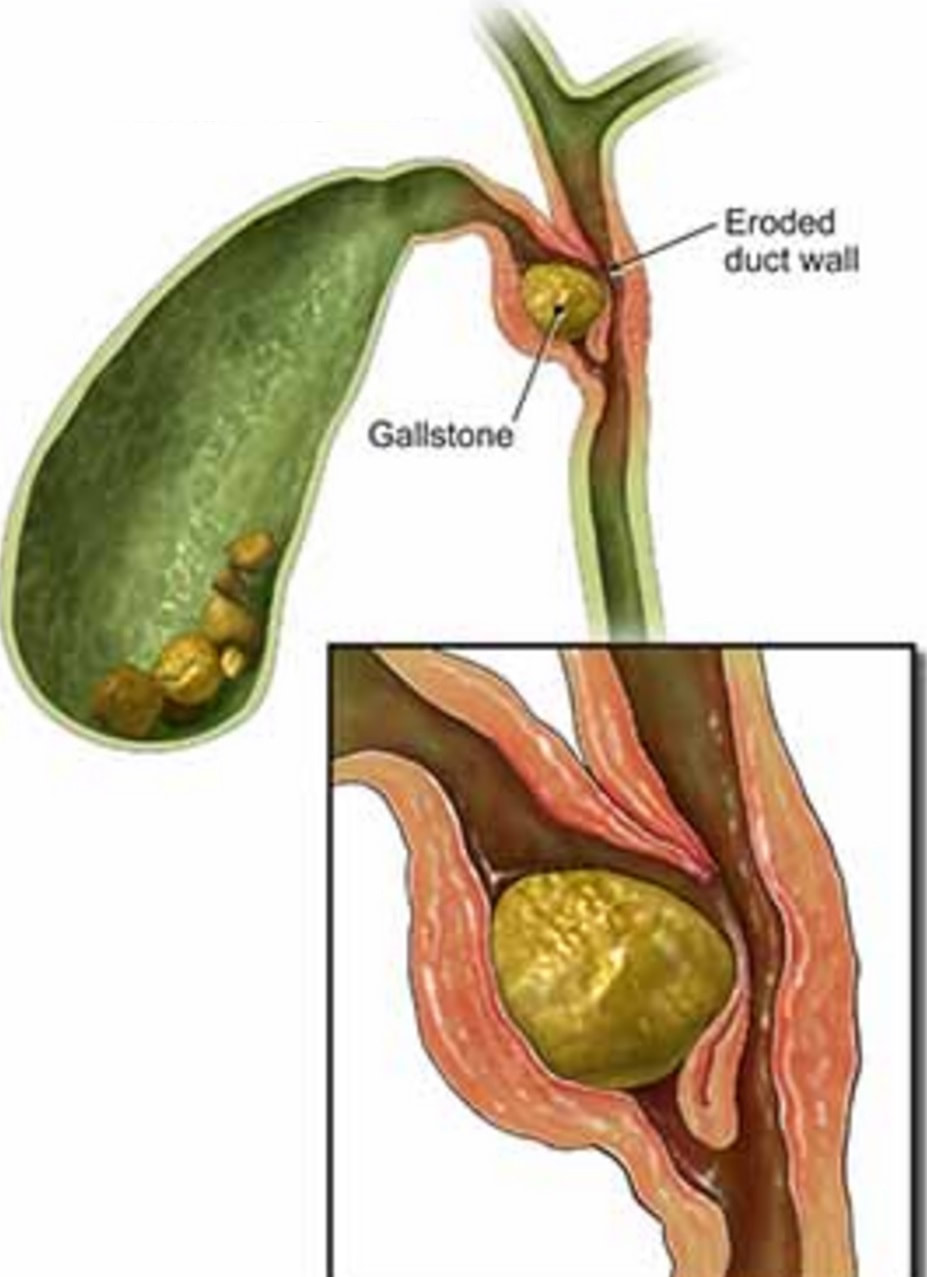
Thanks for sticking with this case until the end! Actually, you did have an opportunity of getting the diagnosis when you heard that the gallbladder histology showed; "markedly thickened wall but with only mild inflammation of the gallbladder mucosa" ...
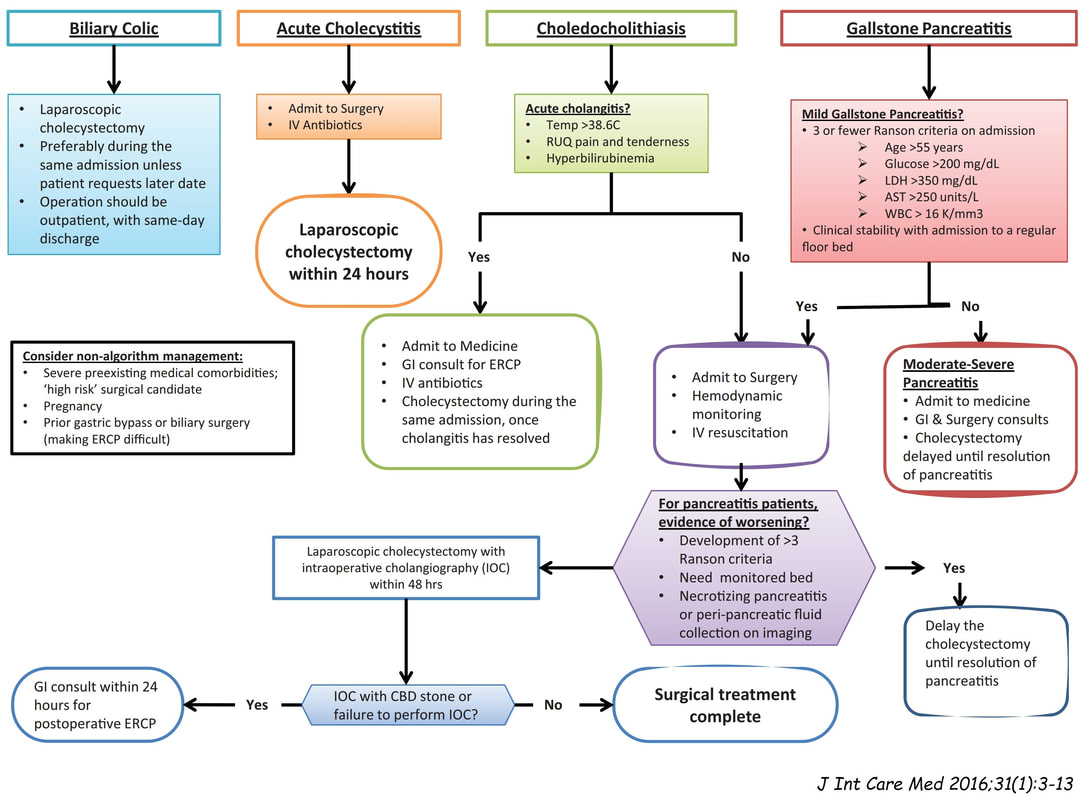
Of course, this doesn't make any sense! In acute cholecystitis there should be an INTENSE inflammation of the mucosa. So why is the gallbladder wall grossly thickened? A second look into the deeper aspects of that thickened gallbladder wall led to a revelation ! The 'second look histology' reported a 'poorly differentiated diffuse type adenocarcinoma deep within the gallbladder wall with single and files of small neoplastic epithelial cells (histology slides below). The pathologists reported that the tumour did appear to be coming from outside the gallbladder. Of course in the video you should have noticed that the gastric antrum was abnormal, a little indurated and thickened. The samples taken from the gastric antrum confirmed the same diffusely infiltrating adenocarcinoma. Those with VERY sharp eyes, would have seen the antral thickening on the initial CT which wasn't commented upon by the radiologist! So what was the final diagnosis? A diffuse type gastric cancer invading into the gallbladder, cystic duct and hepatic duct ! This may a good time to remind you of the management of gallstone related disease. The 'infographic' below from J Int Care Med 2016;31(1):3-13 summarises everything !
This patient presented with a raised ALP. A somewhat plump duodenal ampulla was the only visible abnormality. An abdominal ultrasound demonstrated an dilated CBD but a normal PD. A set of biopsies did not show any abnormalities.
WHAT WOULD YOU DO NEXT?
■ ERCP
Contraindicated as the patient isn't jaundiced or septic!
■ MRI
'Tissue is the Issue'! And an MRI will not give you that!
■ EUS
YES! Because it will allow targeted biopsies of the mass which must be there!
explanation
The ampulla does indeed seem a little plump! There must be something inside it which is pushing it out like this! We organised an EUS which confirmed a nodule inside the ampulla. FNA confirmed adenocarcinoma. As the lesion extended deeper than 5-6mm (on EUS) and because cancer was confirmed, there was no question about attempting an ampullectomy.
As the patient was jaundiced and there was no suggestion of cholangitis, an ERCP would not be indicated. A subsequent MRI also identified a small mass at the bottom end of the bile duct but of course could not provide the tissue diagnosis. |
Categories
All
|