|
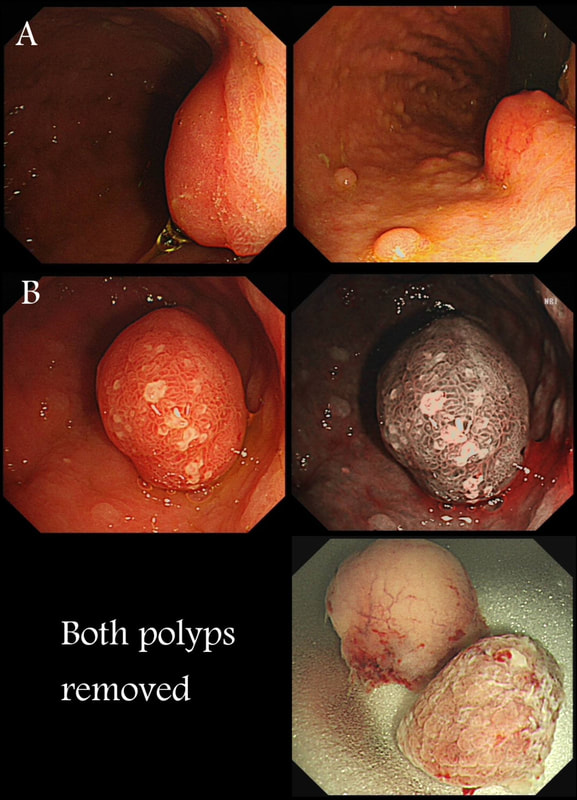
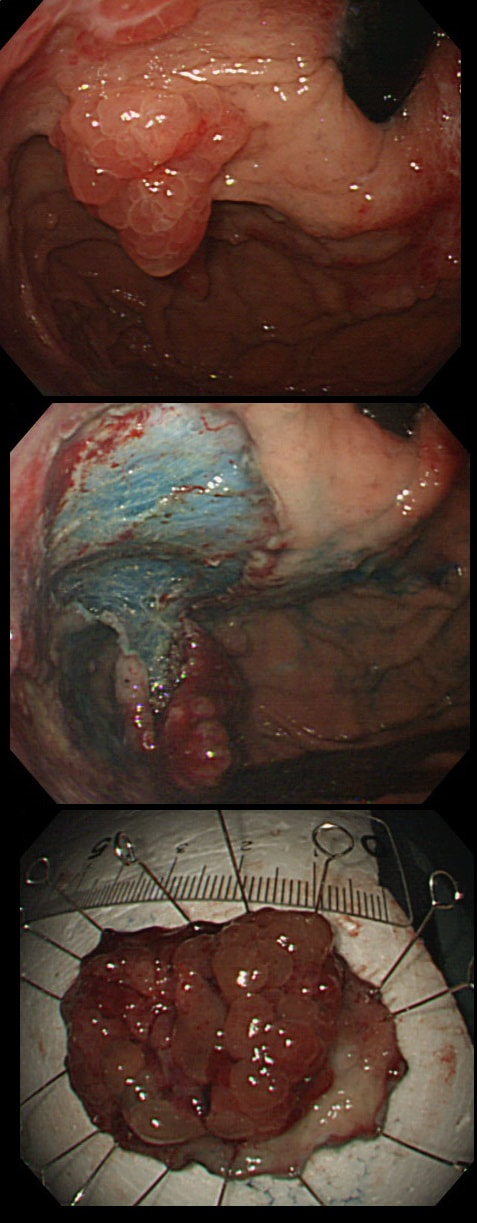
Two gastric polyps (labelled A & B) were both removed from the stomach of a 60 year old woman who complained of indigestion.
WHAT IS THE LIKELY DIAGNOSIS?
■ Both are hyperplastic
But the look different to each other!
■ NET + HP polyp
Well done ! And presumably you know which is which?
■ Both are NET's
But they look different!
■ HP + Adenoma
How about those fine vessels seen in the second image?
■ Both are adenomatous
But they look different!
explanation
You can see the thin vessels crawling up the side of polyp A. This appearance is typical of a gastric NET. Polyp B has a more villous surface with a few white spots - all typical of a hyperplastic gastric polyp. Some of these may contain some malignant cells which somehow generate a hyperplastic/reactive/inflammatory reaction around themselves. However, most hyperplastic gastric polyps are benign and arise secondary to a Helicobacter pylori gastritis. For this reason, you should always do a CLO test in these cases.
Every few weeks, I look up the notes on Prof Pritchard's' Podcast on gastric NET's to remind myself of the workup of these cases. As you remember, you should take samples to confirm an atrophic gastritis whenever you find a gastric NET. In this case, the patient did NOT have an atrophic gastritis. Instead, there was a Hp associated gastritis which is the reason for the second polyp. We realised that what we had was a TYPE II gastric NET! Analysis confirmed that the 17mm was WHO grade II. The finding of anything else than an innocent Type I gastric NET means that further imaging was required. A little late in the day of course but fortunately, the following imaging investigations were unremarkable:
Anyway, below is a reminder of what to do at gastroscopy, when you have a case of gastric NET:
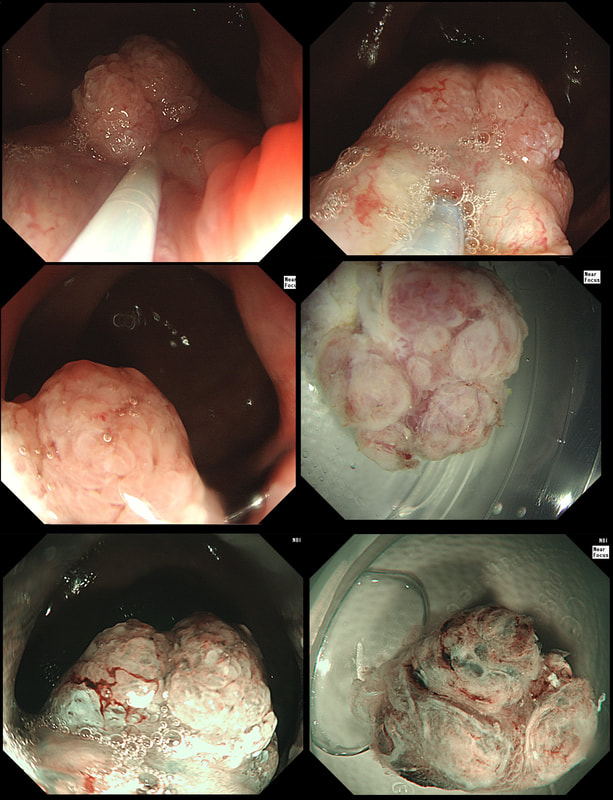
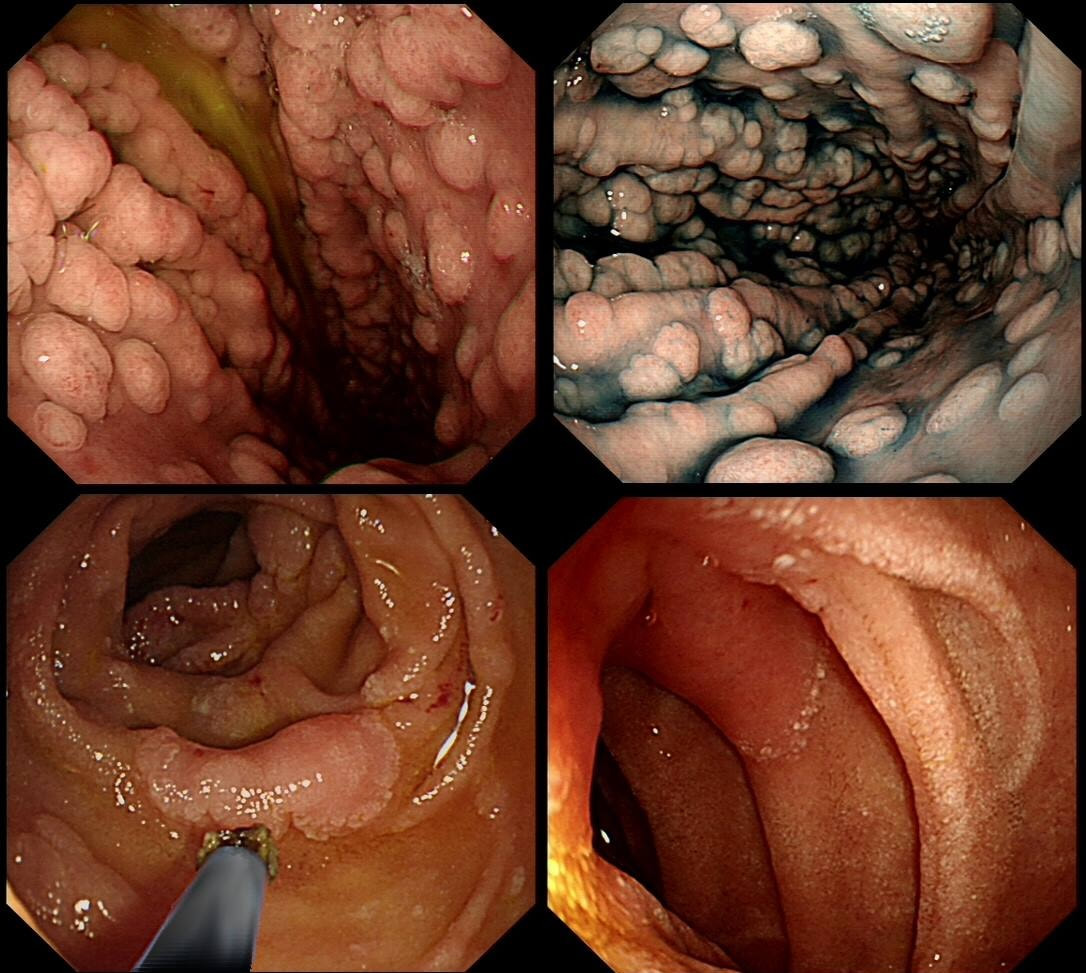
These three polyps were removed en-bloc from the proximal colon. In an earlier examination 2 SSL's had been removed. A subsequent colonoscopy finds no further polyps. WHAT WOULD YOU ADVISE AS REGARDS SURVEILLANCE?
■ There is no need for surveillance
You may be right if the patient is old and with multiple comorbidities!
■ Surveillance every 2 years
Yes, this is what guidelines usually recommend for 'SPS'
■ Surveillance every 5 years
Too long if pt fulfills WHO criteria for SPS
explanation
If course, this patient has 'Serrated Polyposis Syndrome'. It's important to recognise that the serrated polyp count is cumulative over multiple colonoscopies. The recently updated 2019 WHO criteria for Serrated Polyposis Syndrome recognize two types of the syndrome: a 'proximal phenotype' with serrated polyps proximal to the rectum, all being ≥5 mm in size, with at least two being ≥10 mm in size (criterion I 2019), and a more 'distal phenotype' with more than 20 serrated polyps of any size throughout the large bowel (criterion II 2019) [Gastroenterology 2020;158:1520–23]. Personally, I believe that there are more than two subtypes of the Serrated Polyposis Syndrome. There is accumulating evidence that the syndrome includes multiple conditions with variable phenotypes and with different risks of progression to CRC [Gut 2010;59:1094–1100]. This would explain the huge range of cancer risk (25%-70%) in published studies [GIE 2016;83:563–65]. Of course, the likely mixture of several 'syndromes', makes writing guidelines difficult. A recent consensus update by the US Multi-Society Task Force recommend offering a follow-up colonoscopy to average risk patients based on number and size of the SSL's found [GIE 2020;91:463–85]. Interestingly, the US guidelines make a distinction between 'hyperplastic polyps' and 'sessile serrated polyps' although pathologists can't reliably make that distinction. Furthermore, the guidelines excludes patients with an increased life-time risk of cancer which of course excludes patients with Serrated Polyposis Syndrome. I find it all somewhat confusing! James East's BSG guideline 2017 [ Gut 2017;0:1–16 ] recommend surveillance every other year whilst the more recent BSG/ACPGBI guideline of 2020 [ Gut 2020;69:201– 23 ] would seem to suggest 3 yr for all 'high risk cases. But these guidelines expressively don't cover pts with hereditary cancers. Hereditary cancers are instead covered by the BSG/ACPGBI 2019 guideline [ Gut 2019;0:1–34 ] which recommends annual surveillance until all polyps are cleared and then every 2 years. Finally, I admit that I also take the age of the patient into consideration as well as the presence of both serrated and adenomatous polyps. A 40 year old person is surely more likely to benefit from surveillance than a 75 year old person with multiple comorbidities? In particular, I would worry about a young patient, perhaps 35 year old, with 1-2 large serrated polyps and perhaps only a single adenoma. Current guidelines don't flag these individuals up but personally, I would organise another surveillance colonoscopy in a few years time. Clearly, more research is needed to unpick the different serrated sub-pathways ! These polyps are found in a rectal pouch WHAT IS THE UNDERLYING DIAGNOSIS?
■ Serrated polyposis syndrome
Polyps is SPS are flat (usually)
■ FAP
Looks like a classic example of rectal surveillance in FAP but polyps do look odd don't they?
■ Juvenile polyposis
Yes! Pt had already developed a CRC and after a subtotal colectomy offered rectal surveillance
explanation
These polyps have a funny crypt pattern, but THERE IS A PATTERN! Accordingly, they are likely to be 'hamartomatous' (a non-sensical histological term which basically means overgrowth of normal tissue). Of course, this patient has Juvenile Polyposis syndrome. Although the polyps are 'hamartomatous', as in Peutz-Jeghers syndrome, there is a stark difference. The polyps in JP often turn dysplastic and are presumably the origin of this patients adenocarcinomas. In contrast, the polyps in PJS are extremely rarely reported to harvest dysplasia (I've never seen a dysplastic polyp in PJS). By the way, the WHO criteria for diagnosis of juvenile polyposis syndrome are one of either:
A very odd looking polyp at the anal verge. I removed it but still couldn't really make out what it was!?
WHAT IS YOUR BEST GUESS AT THE MOST LIKELY DIAGNOSIS?
■ Inflammatory polyp
Good guess as there is no crypt pattern!
■ Adenomatous polyp
It has the colour of an adenomatous polyp but no crypt pattern...
■ Small adenocarcinoma
Certainly ugly enough but small carcinomas are usually more button-shaped
■ Small SCC
Arising from close to the anal margin but nearby squamous mucosa is unremarkable...
explanation
This is rare stuff! I was clueless although reassured that the thing was soft and didn't feel malignant when squeezed between my fingers.
It proved to be a (rare) 'cloacogenic polyp'! You'd be excused if you have never heard of this (I hadn't). Apparently these were first described as recently as 1981! The same year that I was born ☺! Essentially they are inflammatory polyps arising from the transitional zone of the anorectal junction. They can be much larger than this and even be multiple and can occur at any age. Patients are usually asymptomatic but could (naturally) present with some blood on the toilet paper (this patients complaint). Histology is similar to that of solitary rectal ulcers with inflammation and fibromuscular hypertrophy. For this reason it is perhaps not surprising that they are linked with constipation and straining. Rare associations include small nearby cancers and human papillomavirus infection.
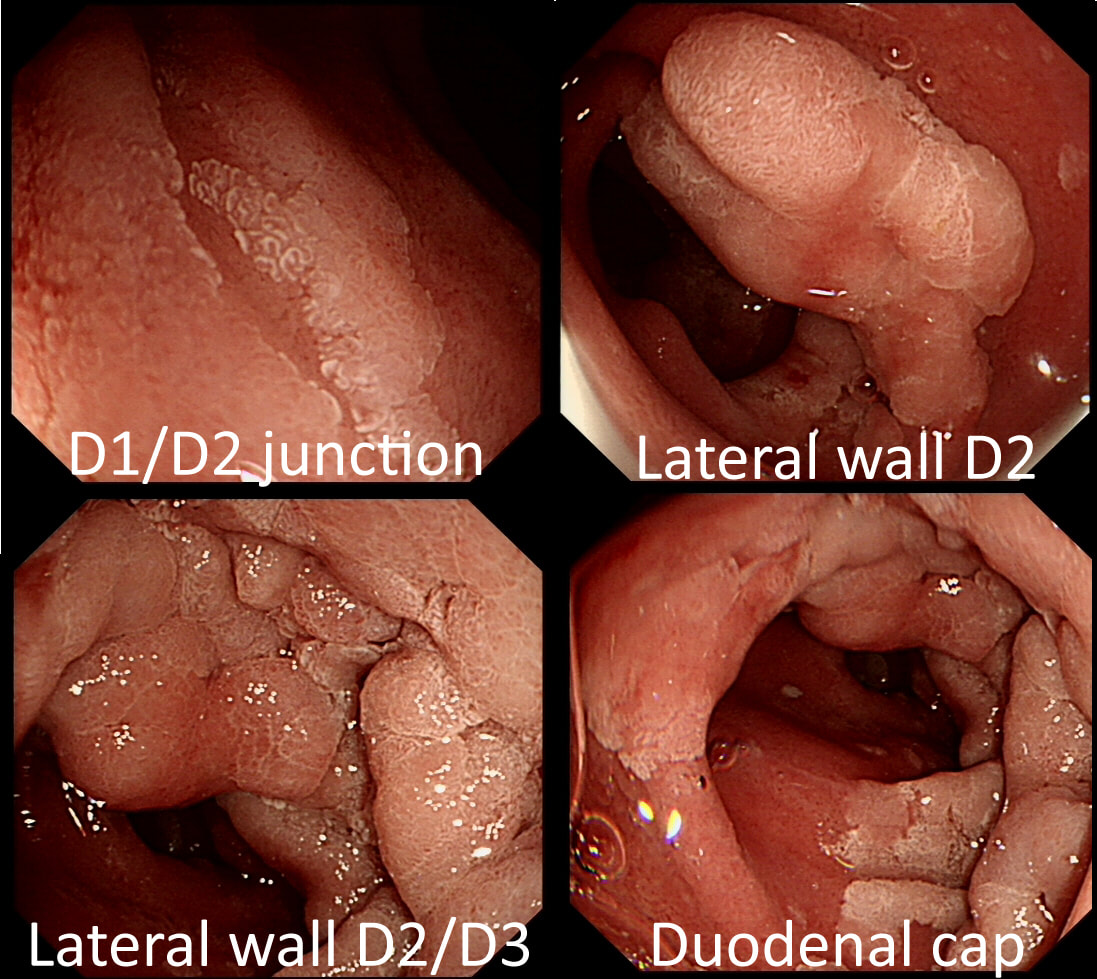
A fit middled aged patient with FAP is referred for consideration of a duodenal EMR for his duodenal polyposis. Earlier samples have indicated that this is a villous adenoma harbouring up to high-grade dysplasia (VA+HGD).
SHOULD WE ABORT OR ATTACK?
■ Abort and refer surgically
Yes, my inclincation as well
■ Attack!
Hm, what is that Spigelman score?
explanation
The Spigelman score is a little peculiar in that patients with scores of 0 to 3 have a very low risk of developing cancer. However, when patients accumulate a score of 4, surgery should be considered as there is a high risk of cancer.
Of course, many patients with FAP have desmoids which precludes surgery. In those cases I consider intervening endoscopically if: 1) the patient is fit enough to survive emergency surgery to sort out a perforation or cope with a 4+ unit blood loss AND/OR 2) there is a higher than average risk of cancer, for example in a patient with extensive HGD or confirmed IMca. Of course, the lesion also has to be 'resectable' within your level of expertise. Personally, I find lesions situated at 12 O'clock the most challenging to remove. Furthermore, a general anaesthetic is probably a prerequisite for resections taking longer than 20 min or so (which they always seem to do). Thus, I consider the "correct" answer to be 'Abort'! I decided that the lesion was likely to be too large for me to resect. Fortunately, the patient was a good surgical candidate. A 'pancreas sparing duodenectomy' was carried out and a TVA with mainly LGD but also areas with HGD was completely removed. Postoperatively the patient developed pancreatitis and was discharged 4 months later... Clearly this is 'Tiger Country' for surgeons as well !
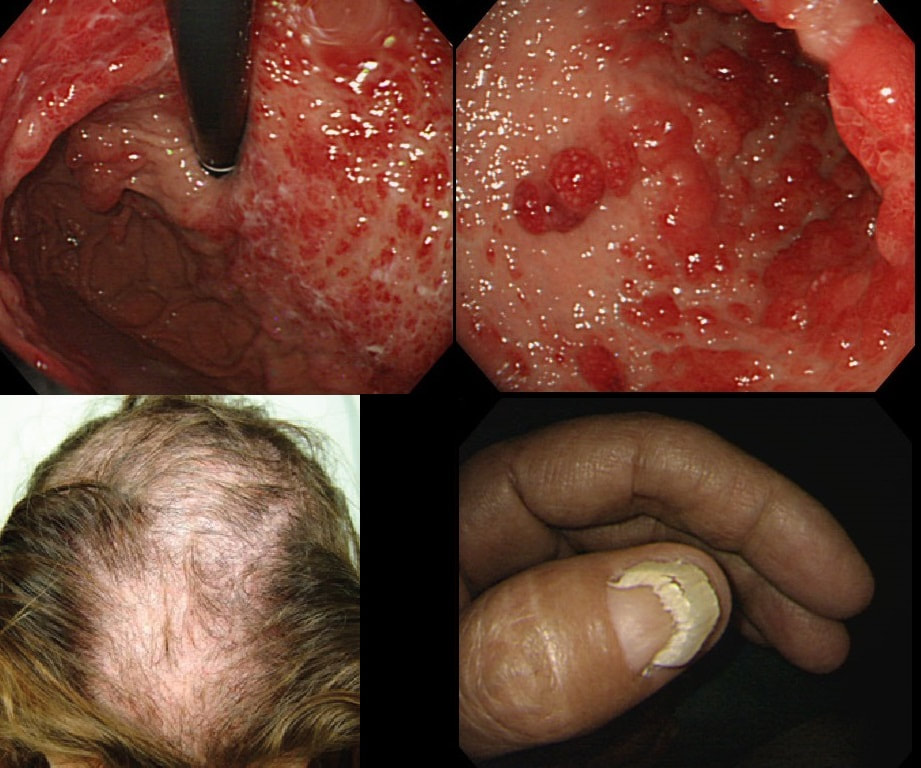
You are called into another endoscopy room to give an opinion on this gastric findings. It's a 65 year old man undergoing investigations for severe weight loss and anaemia. The anaemia was discovered by the dermatologists where he had been referred for investigations of suspected onychogryphosis and thinning of the hair.
WHAT IS THE EXPLANATION FOR THE GASTRIC POLYPS?
■ Likely hyperplastic polyps
INCORRECT!
■ Likely hamartomatous polyps
CORRECT! Clearly you know what the underlying diagnosis is?
■ Likely florid GAVE
INCORRECT!
■ Likely lymphoma
Gastric Lymphomas are usually ulcerated
■ Likely linitis plastica
INCORRECT! (although I guess it could be)
explanation
The nail dystrophy is not due fungal infection (onychogryphosis). In fact, this patient has Cronkhite-Canada syndrome. This enigmatic acquired syndrome is characterised by malabsorption, gastrointestinal polyposis, nail dystrophy, alopecia, cutaneous pigmentation, diarrhoea and weight loss. The nail dystrophy and skin pigmentation is clearly seen in the photograph. Other common symptoms include abdominal discomfort and a protein loosing enteropathy. The underlying cause is unknown. The cutaneous manifestations are probably all secondary to severe malnutrition due to diffuse small bowel mucosal involvement.
Histologically the mucosa is oedematous with dilated glands and an inflammatory cell infiltrate of the lamina propria. Gastrointestinal polyps are hamartomatous but may contain foci of adenomatous epithelium. The histopathologists will struggle in interpreting the biopsy findings and will mention things like 'marked foveolar hyperplasia', 'cystically dilated irregular glands', 'oedematous stroma' and 'scattered mixed inflammatory cells'. The differential diagnosis of such hamartomatous findings includes juvenile polyposis syndrome, Cronkhite-Canada syndrome, hyperplastic polyposis and Menetrier's disease. Of course, it will help them to know about the nails and hairloss etc!!! Malignant transformation has been reported and may not be as rare as initially thought . The prognosis is said to be poor but I'm not sure that this is still the case with 'modern management'. Interestingly, in this case a suspicious nodule was found (image below). It was removed endoscopically and found to be an early gastric cancer!
This was found at Gastroscopy in a middle aged man without any family history undergoing gastroscopy because of an iron deficiency anaemia.
WHAT WOULD YOU DO NEXT?
■ Take a full set of biopsies
INCORRECT!
■ Organise and EMR
INCORRECT
■ Request a capsule study
INCORRECT!
■ Organise a colonoscopy
ABSOLUTELY!
■ Request a CT abdomen
INCORRECT!
explanation
There lots of "cystic fundic polyps" and there is also at least one duodenal adenoma. Such findings in a 55-year-old man is suggestive of the "attenuated" form of familial adenomatous polyposis (FAP). In the absence of a family history, presumably this is a new mutation. Of course this means that the most likely reason for the iron deficiency anaemia is a colorectal cancer. This patient needs an urgent colonoscopy!
To remind you, the APC gene is located on the long arm of chromosome 5 and encodes a tumour suppressor protein. APC is a huge protein that no doubt acts in many different ways to help control cell division and cell attachment and preserve the chromosome number through cell division. Normally, APC mops up intracellular ß-catenin. The newly formed “APC– ß-catenin complex” is quickly destroyed. The removal of intracellular ß-catenin is a good thing! Because when a mutated APC gene is less capable of mopping up intracellular ß-catenin, cell-to-cell adhesion is reduced and cells are allowed to stay non-differentiated and immature. That's not good! Of course there is a second possibility. This could also be a new MYH gene mutation. Briefly, the MUTYH gene encodes 'MUTYH glycosylase', which is a DNA repair enzyme. Similarly to attenuated FAP, patients with MUTYH gene mutations develop multiple colonic polyps in adulthood. However, in contrast to attenuated FAP, patients with MUTYH gene mutations have both an markedly increased risk of both colonic and gastric cancer. Characteristics of 'attenuated FAP' compared with 'classic FAP' are:
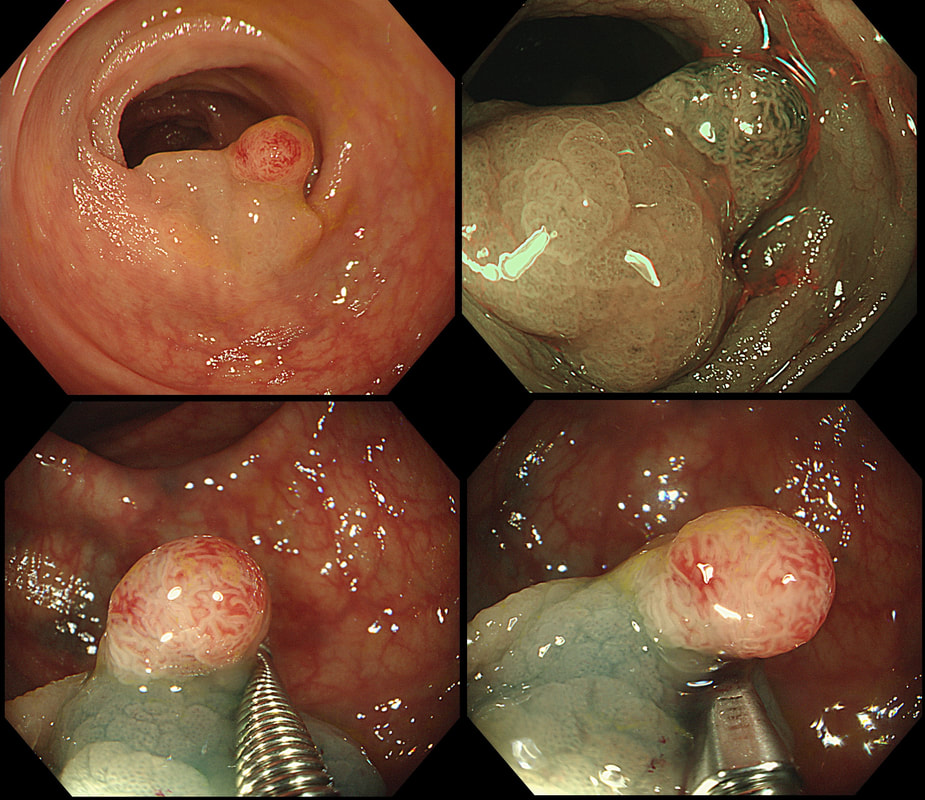
This polyp was found in a 30 year old patient with a family history of bowel cancer undergoing colonic surveillance because of a 'polyp syndrome'.
WHAT IS THE LIKELY POLYP SYNDROME?
a) Familial Adenomatous Polyposis (FAP)
INCORRECT!
b) Peutz-Jeghers syndrome
INCORRECT!
c) MUTYH associated polyposis (MAP)
INCORRECT!
d) Serrated Polyposis Syndrome)
CORRECT!
e) Lynch Syndrome (formerly HNPCC)
INCORRECT!
explanation
This proved to be a difficult question and only 15% of our facebook group got the correct answer of: Serrated Polyposis Syndrome !!!
The odd thing about this polyp is that it appears to be a tubular adenoma (slit-like crypts) arising from a broad somewhat odd looking fold. The 'fold' is actually a larger hyperplastic polyp. Of course large hyperplastic polyps are now referred to as 'sessile serrated lesions' (SSL's). You are looking at an SSL, sprouting a TA !? A 'purist' histopathologist may refer to this entity as a 'mixed serrated, adenomatous polyp'. Do you know of a polyp syndrome where patients have a mixture of SSL's and adenomatous polyps? Of course, the serrated polyposis syndrome 'SPS' (previously called the sessile serrated polyposis syndrome). It's now realised that many patients with serrated polyposis syndrome actually have a mixture of serrated and adenomatous polyps. It's possible that these patients have a greater risk of developing cancer. The correct answer is therefore 'A' ! The BSG has published a 'position statement' on the topic of SSL's which is well worth reading. The BSG sensibly recommend annual surveillance until all serrated polyps above 5mm have been cleared, following which the surveillance interval may be reduced. This is because patients with SPS do not seem to have a higher risk of developing cancer than patients with Lynch syndrome. |
Categories
All
|