|
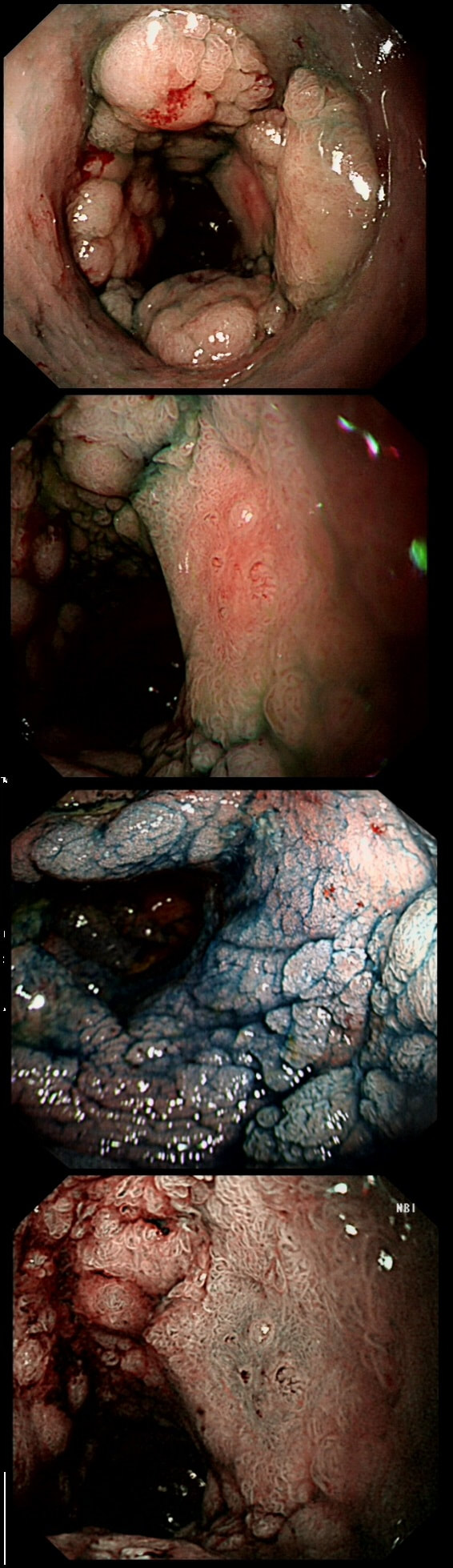
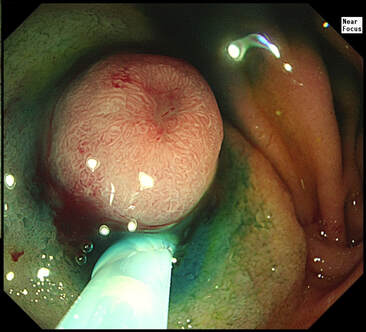
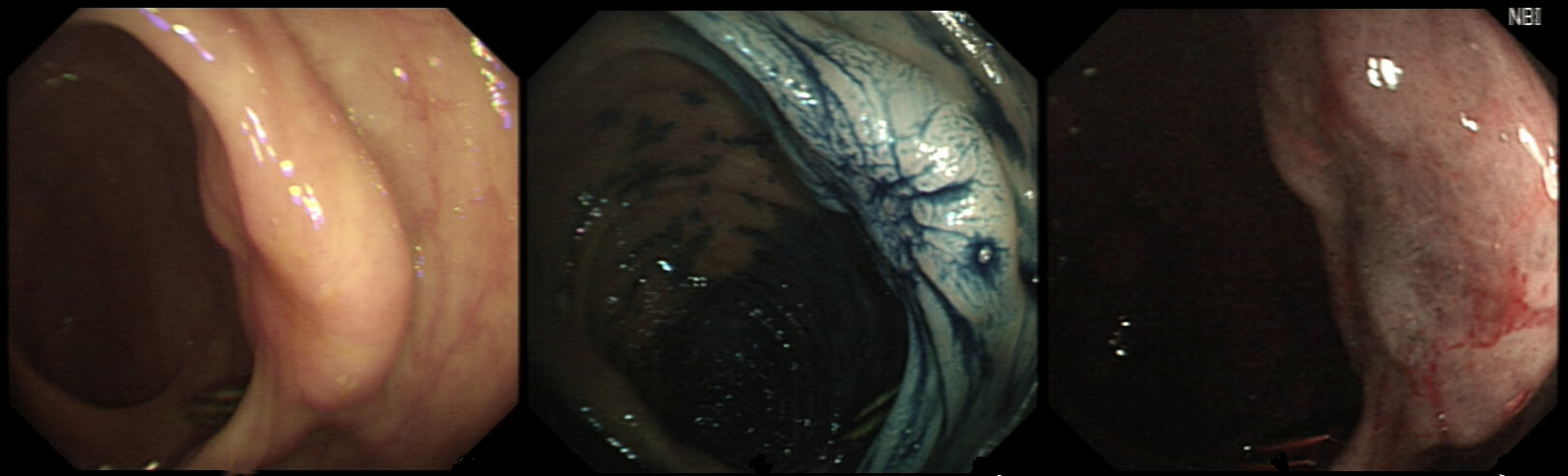
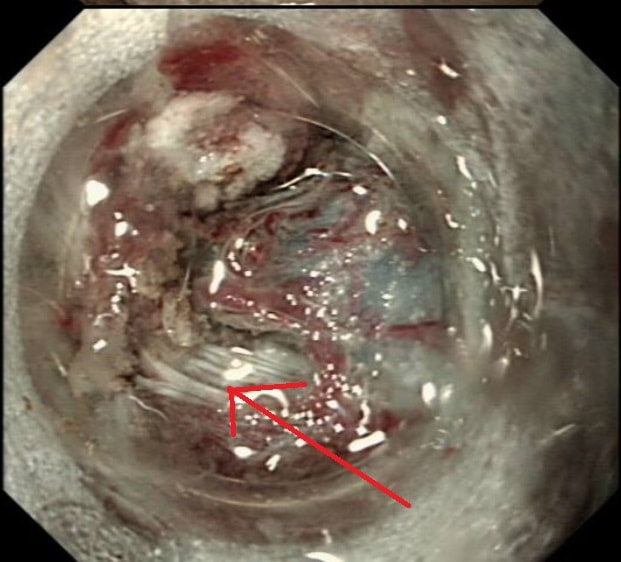
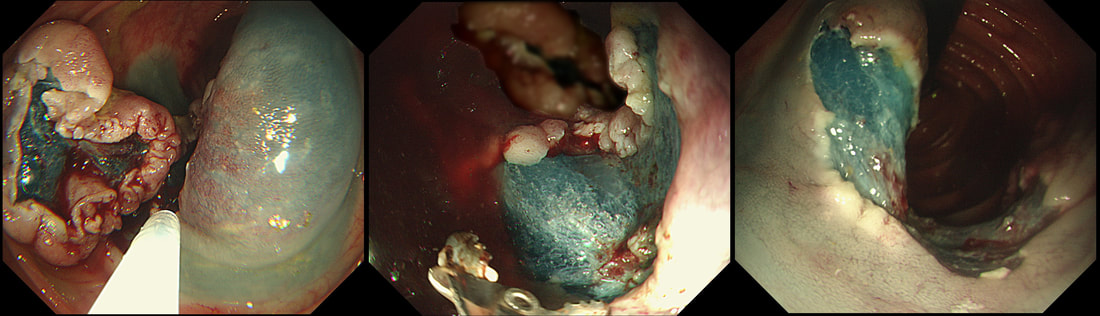
This is a video clip of a small lesion removed from the sigmoid colon. WHAT IS THE MOST LIKELY HISTOLOGY?
a) TA+LGD
But it's a IIc lesion with IIIs crypts in the centre?!?
b) TA+HGD
Absolutely!
c) Invasive cancer
But there ARE small round crypts in the centre and it DOES lift!!!
explanation
You may call this a "flat elevated lesion with a central depression (IIa+IIc lesion) or simply a depressed lesion (IIc lesion). Frankly it doesn't matter because both a part of the same 'family' of evil little b.....ds. They are always TA's and the small, round crypts (Kudo type IIIs crypt pattern) tells you that the lesion harbours HGD. This is because as dysplasia progresses from low to high grade, crypts get smaller and more withered. Of course they eventually disappear altogether as the lesion develops into a cancer which no longer follows any 'instructions' to form organised crypts. However, the crypt pattern is still discernible in the centre AND the lesion lifts well. Both of these tells you that the lesion is likely to still be benign. Ultimately, the pathologists called it a TA+HGD. However, there was mucinous differentiation in the centre of the lesion. Could these little shits be the early stage of mucinous colonic cancers? Quite likely! Imagine how easily they are missed when hiding behind a fold or below a shallow puddle !!!
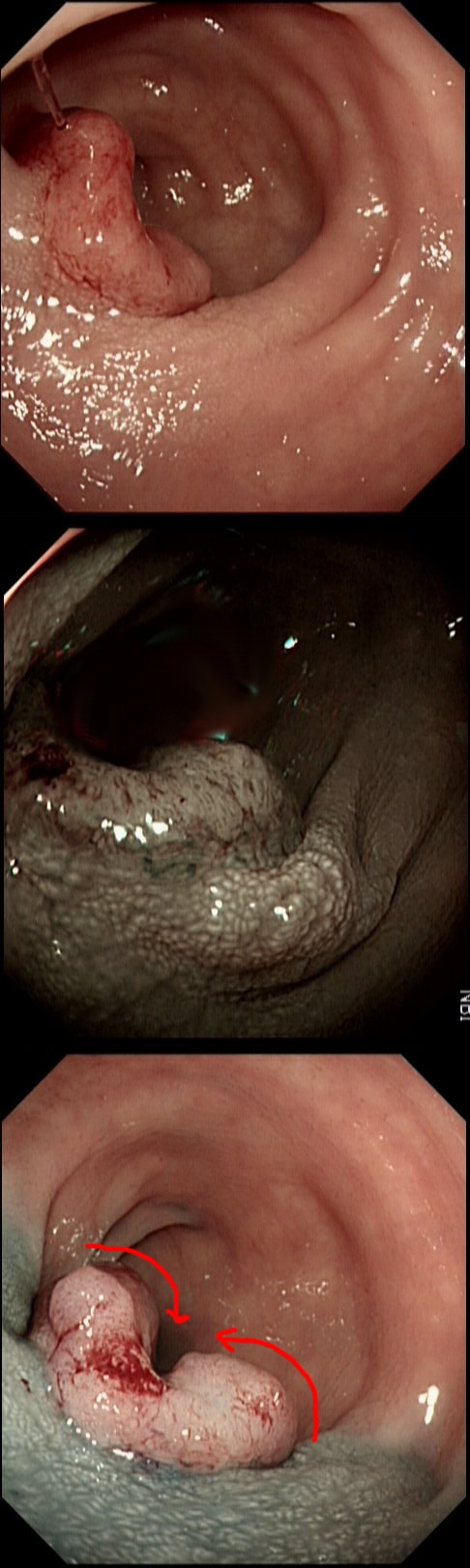
This patient with ulcerative colitis has developed a polyp in the transverse colon. The lesion has now been sent for an endoscopic resection.
WHAT WOULD YOU DO?
a) Abort!
Smart!
b) Attack!
You are creating a problem of your own making!
explanation
Some would say; "if you can remove the lesion in that colitic colon, then 'do it'! The problem is that nothing may appear "irresectable" giving plenty of time, determination and poor judgement.
Many studies looking at outcomes of polypectomy in UC, excluded polyps >1-2cm or flat polyps. Other studies have included polyps arising outside of the colitic field or only have a short follow up period of a few years. Actually, most are coming to believe that when dysplasia develops in the colitic colon, it's not a 'random' case of bad luck. It can be the result of a long process of progressive DNA damage! At some stage we will be able to have a look at the state of the stem cell DNA in patients with conditions such as Barrett's, Colitis and atrophic gastritis. I think that we are in for a surprise ! In addition, did you spot the small focus of invasive cancer in the 2-3 O'clock position? Surprisingly, this was only T1 disease!
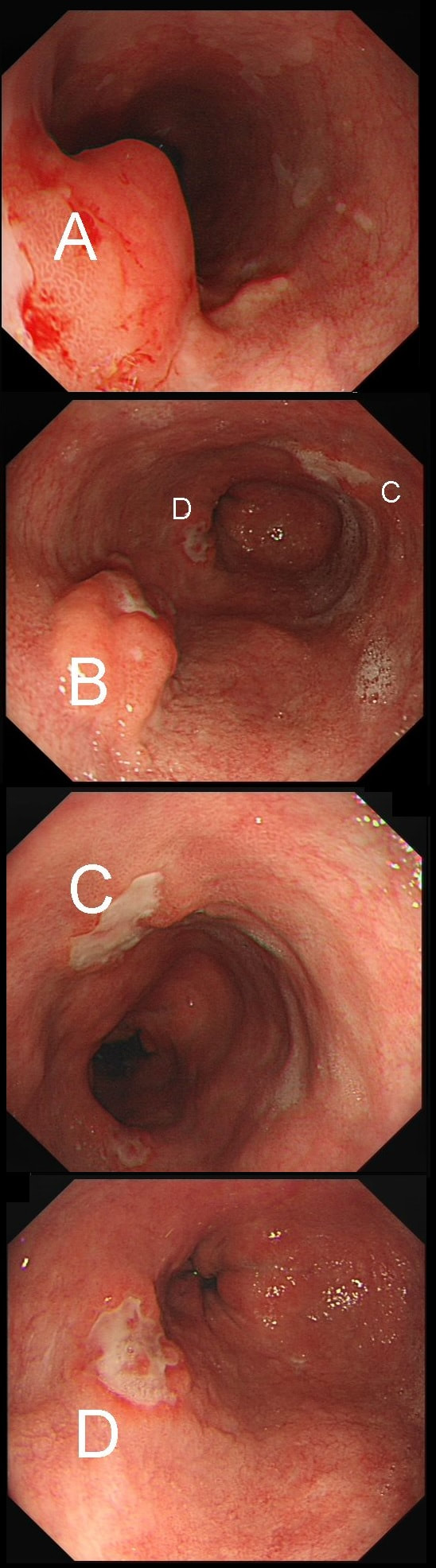
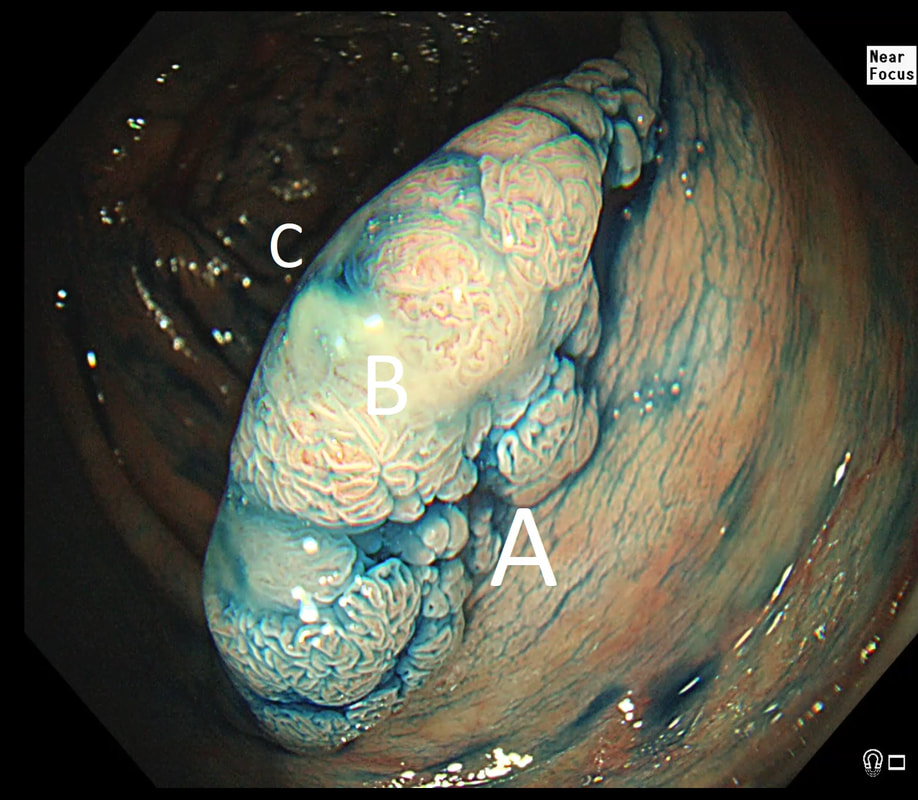
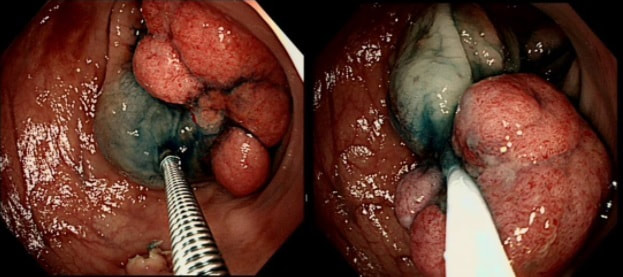
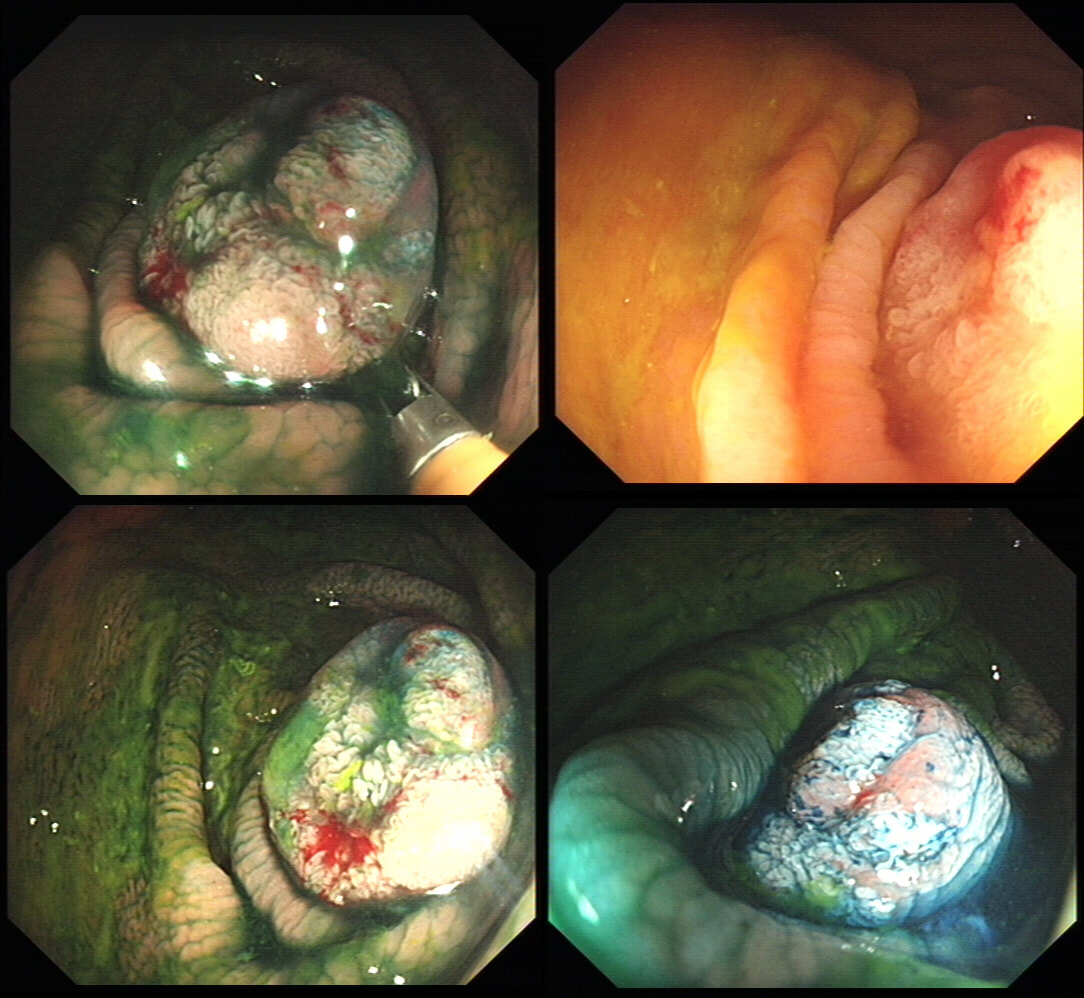
55 yr pt with a Barrett's nodule is referred for an endoscopic resection. I'm surprised to find 4 abnormalities within his 10cm stretch of Barrett's
WHICH OF THESE IS THE MOST LIKELY TO BE ENDOSCOPICALLY RESECTABLE?
a) Lesion A
I agree! Of these 4, A is the only one which isn't ulcerated and therefore is least likely to be invading deeply.
b) Lesion B
Would be my second guess as the ulcerated area seem superficial
c) Lesion C
Don't like the ulceration!
d) Lesion D
Would be my least favourite lesion to attack as the ulceration suggests deeper invasion and poor lift into my cap.
explanation
This may be something of a record, 4 synchronous lesions! Clearly A, B and D are malignant. At first, ulcer C seem more innocent without an elevated edge but on closer assessment, it also has a slightly elevated rim.
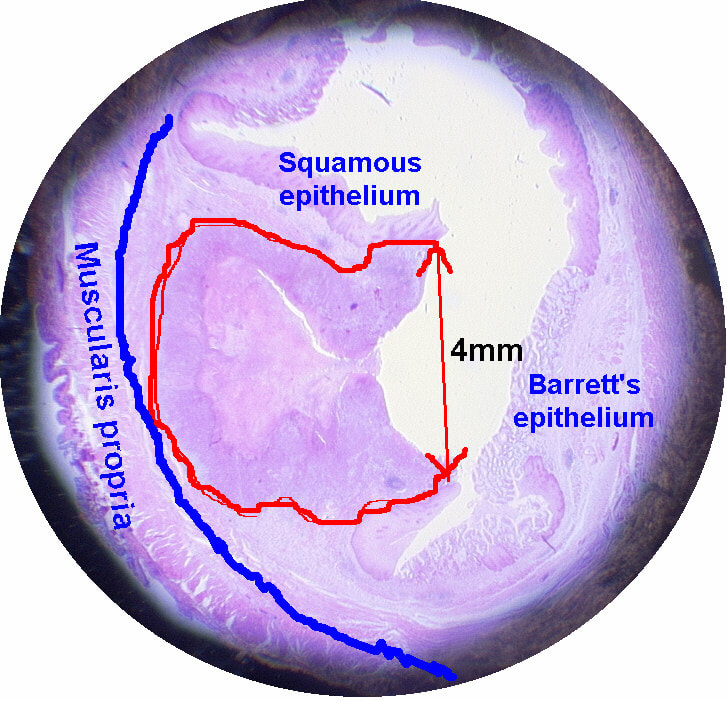
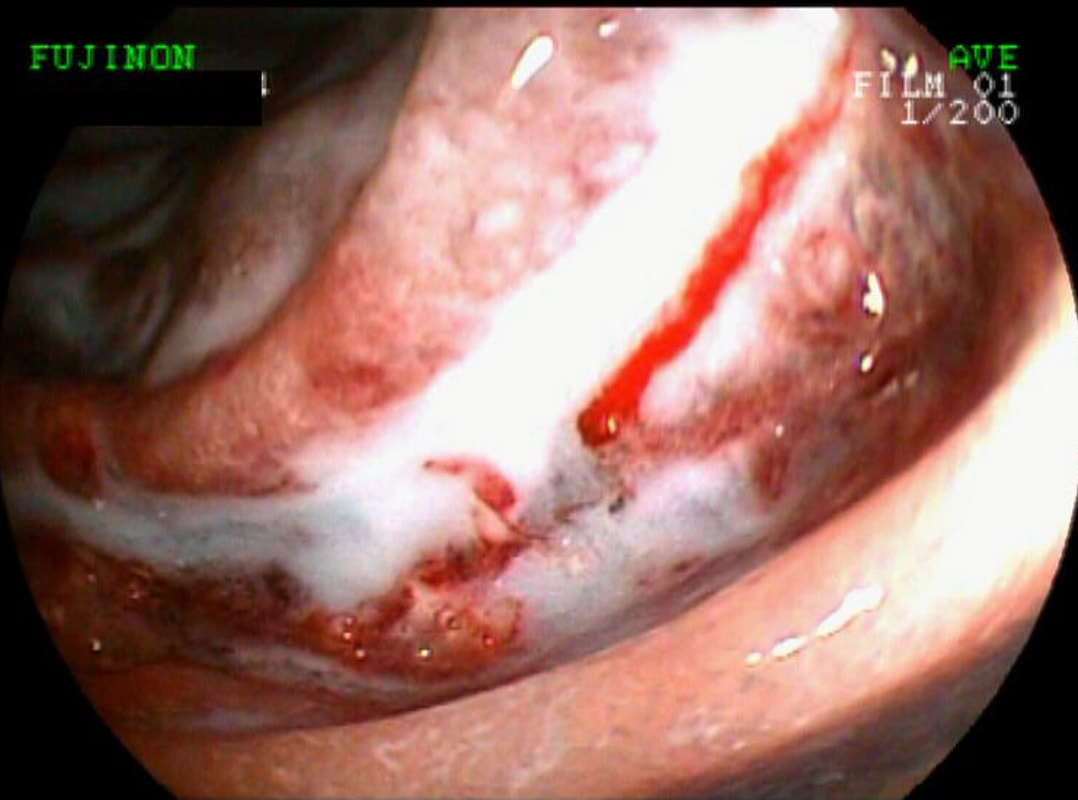
Of these 4, A is the only one which isn't ulcerated and therefore is least likely to be invading deeply. Of course, they are assessed together as there is no point in EMR'ing one only. Either all are curable endoscopic means or none are ! Rather than going ahead with attempting to resect these, I actually bailed out and took samples from each lesion. Biopsies showed invasive, poorly differentiated adenocarcinoma at each location! Clearly, this patient has multifocal 'bad disease' which endoscopy is unlikely to cure in my opinion. I believe that surgery is a far better option and the patient is currently awaiting his oesophagectomy. If you still are not convinced of the pitfalls in trying to deal with ulcerated Barrett's lesions, have a look at the lesion below. Two rounds of sampling had indicated that the lesion harboured HGD. However, I failed to remove the lesion and ultimately the patient underwent an 'Ivor-Lewis'. You can see the histology yourself. The 4mm surface is literally the tip of the iceberg and below you can see the cancer (red line) invading up to the muscle propria.
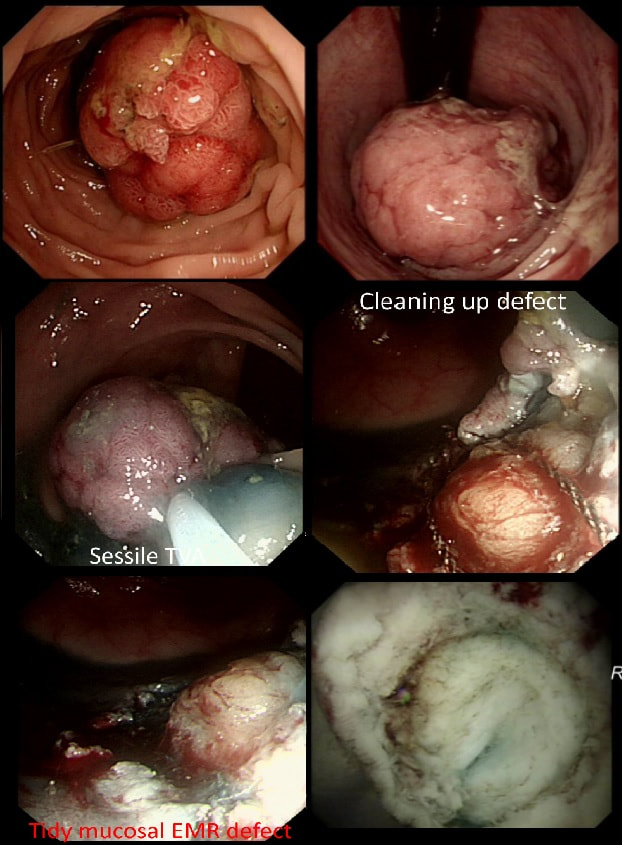
This polyp was found in the sigmoid.
HOW IS IT BEST REMOVED?
■ Cold snare polypectomy
Wouldn't be my first thought!
■ EMR
I agree - single fragment EMR!
■ ESD
Would just add time and expense to procedure (unless you need practise, I guess)
explanation
This 10-12mm polyp is covered with a beautiful gyrate crypt pattern typical of a TVA. The risk of cancer deep within, in spite of the normal surface is far below 1%. But it's not 0% and it would also be a little difficult to cut through this amount of tissue. For these reasons, I think that 'cold snaring' would be wrong.
At the other end of the spectrum, we have an ESD. It would ensure a single fragment resection but would take about 20 minutes or so. The in between the two, we have an EMR. If course, a single injection of 3-4ml would lift this nicely and then a 15mm snare would resect it, single fragment, within a minute. I think that the best method of removal of polyps up to about 2cm, is by EMR as polyps up to this size can usually be removed as a single fragment. This lesion was found in the sigmoid. You have magnification, NBI, dye spray and lift to help you decide. WHAT IS THE STAGE OF THIS CANCER?
■ Intramucosal
There is no such thing!
■ sm1 invasion
You can see a disorganised crypt pattern?
■ sm2 invasion
That was my guess!
■ sm3 or 'massive' invasion
I think that the lift is better than that!
explanation
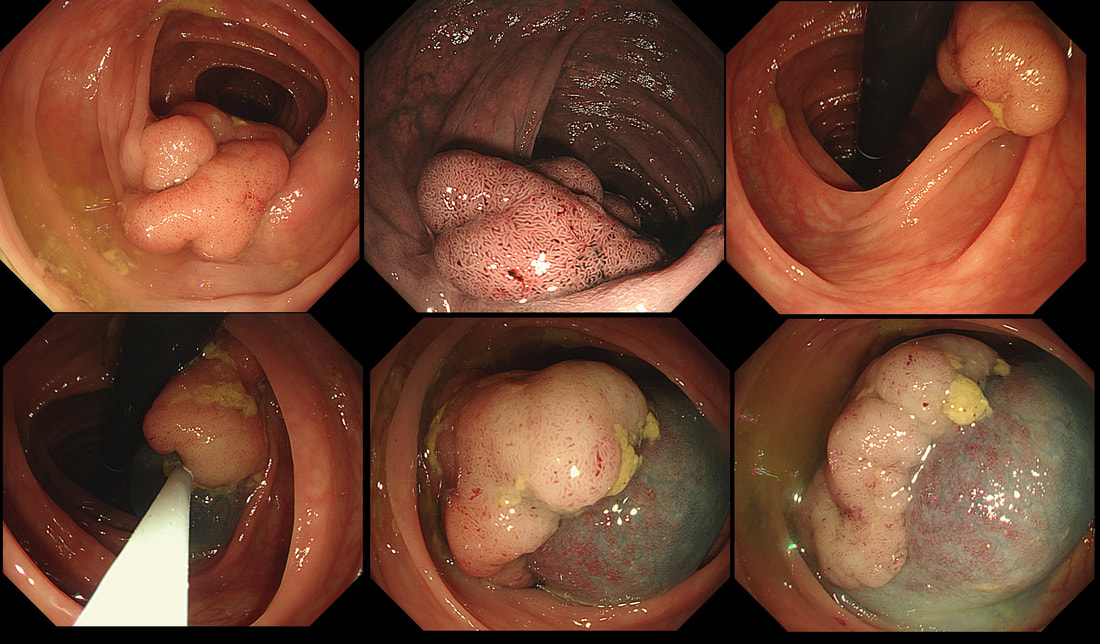
I think that it's difficult to tell the difference between no crypt pattern at all and a severely disrupted crypt pattern. Of course, when the crypt pattern is 'severely disorganised' but some of it is still visible, the lesion will be sm1 or sm2. In contrast, if there is no crypt pattern at all, the lesion is sm3 or beyond (the Japanese call this 'massive invasion'). Instead, I rather rely on the degree of lifting. In this case, the endoscopist decided that the lifting was insufficient for a resection and backed off, referring the patient to our MDT. Biopsies confirmed that the lesion was likely to be malignant and the patient ended up with sigmoid resection. I think that there is some sort of crypt pattern in the centre of the lesion. Furthermore, looking at the slight degree of lifting, my guess would be that the lesion is sm1 or sm2 and therefore potentially endoscopically resectable. Actually the cancer turned out to be sm2 (T1,N0). This is beautiful polyp, perched on a fold, was found at the junction between the caecum and the ascending colon. The video gives you a better idea of the size and extent of the lesion. WHERE WOULD YOU PLACE THE NEEDLE FOR THE BEST 'LIFT' ?
■ On the side facing you
A convenient choice but lift will not be optimal
■ Into the apex of the polyp
That would be my choice!
■ Just behind the lesion
Better than into the fold facing you
explanation
I have no qualms about injecting straight into the middle of lesion provided that I'm sure that it's benign. The lovely gyrate pattern of this polyp tells you that it's a TVA, likely to harbour no more than LGD. Injecting into the fold facing you is likely not to raise the 'blind side' of the polyp which extends down the back of the fold and onto the 'flat' beyond. Conversely, injecting into the back of the lesion would lift the back end but probably not the front. My choice was to inject into the apex (see video below) which resulted in a lovely lift. But, why not inject in two places? Because you should try to avoid injecting into more than one place (if it can be avoided). If you have made more than one hole in the epithelium you may will find that your injection leaks out through the previous hole and that the elevation is less effective. Of course when removing large, flat lesions multiple injection sites can't be avoide, unless you are removing the lesion by ESD of course.
This sessile polyp was found in the caecum, close to the appendiceal orrifice WHAT IS YOUR DIAGNOSIS?
■ Mixed adenomatous & serrated polyp
I don't see a mixture of crypt patterns!
■ SSL
Absolutely!
■ TSA
No, TSA's look like a cross between TVA & VA!
■ Adenomatous polyp
Perhaps the crypts look a little slit-like but it's covered with mucus...
explanation
Well, perhaps the crypt openings look a little slit-like but the lesion is partially covered with that mucus typical of an SSL. By the way, couldn't it be a hyperplastic polyp? I think that pathologists have given up even attempting to tell the two apart! My own rule of thumb is that anything which is ≥10mm, I call an SSL and remove. By the way, SSL's usually lift very well. In this particular case, the lift is a little sub-standard for an SSL, presumably because it's situated very close to the appendix orifice which anchors it down. A word of caution! I've had several 'post polypectomy syndrome' cases after removing large SSL's. In these cases, the lift was excellent and I asked for the LARGE snare to remove the lesion en-bloc. However, in both cases, I found that the snare was taking quite a long time to cut through. Perhaps because of that fatty reaction in the stroma below which Neil Shepherd talked about in the Podcast. To avoid any risk of the 'post polypectomy syndrome', you should ask your assistant to close the snare as quickly and hard as possible. Don't worry about bleeding. These lesions are never supplied by any significant vessels. Oh yes! And place clips !!! This polyp just distally to the caecum has been referred after an initial set of samples have indicated that it's a benign TVA harbouring no more than LGD. An unfortunate, fixed loop at the hepatic flexure means that I can't retrovert behind this polyp. In fact, it's difficult to get much closer to the lesion than this. WHAT STATEMENT DO YOU AGREE WITH?
■ The resection may be difficult but it can be done
I agree with that!
■ The location of the lesion and difficult colonic configuration probably makes a resection impossible
Of course the patient may have to travel to a nearby centre with the neccessary expertise!
■ All this is irrelevant as the polyp looks suspicious and would be best removed surgically
I disagree, it does look benign! But will of course have a 15% risk of harbouring cancer...
explanation
Actually, the lesion does look very benign. It wobbles about freely perched on top of the first fold distally to the caecum. Furthermore, it has a reassuring gyrate crypt pattern of a TVA. Naturally, any polyp of this size will have a 15% risk of actually containing an unexpected focus of cancer within. Because our therapeutic waiting list is in mess in the aftermath of Covid, I do encourage everyone to sample the sessile lesion even though you are planning to refer it for resection. Such sampling on the surface of large sessile lesions doesn't induce any fibrosis and provides some (limited of course) reassurance that the lesion isn't malignant. In this case, the position is very challenging but you can get close enough to stand a good chance of removing the lesion. That's actually what I did and I've just uploaded a video of the resection together with my face-to-camera annotation to highlight the main points. Ultimately, histology confirmed a TVA harbouring no more than LGD This lesion was found at the top end of the colon. WHAT WOULD YOU DO NEXT?
■ Lesion is benign but it can't be removed endoscopically
Best to admit defeat than to fight against overwhelming odds
■ Lesion is benign and I will organise an EMR
Not sure that anyone could remove this endoscopically
■ Lesion is benign and I will organise an ESD
Ridiculous!
■ Lesion is benign and I will organise an underwater resection
It will also fail !
■ Lesion is malignant and requires a surgical resection
Polyp is large BUT looks benign!
explanation
This is a classical LST-G (laterally spreading tumour of the granular type). These lesions are always TVA's and almost always harbour LGD. Unfortunately, there are three problems which precluded an endoscopic resection; 1) there is a crazy alpha loop in the transverse colon which as usual proved impossible to remove, 2) the movement of the diaphragm continuously moves the lesion away and towards you and 3) the main part of the lesion is in the ascending colon but then extends across to the caecum where it almost kiss the appendix orifice. The moral of the story is to carefully consider BEFORE you start the resection. After you have started to attempt resecting a lesion such as this it becomes progressively more difficult to stop! It's like a gambler finding it increasingly difficult to walk away from the table as losses stack up. Because you have invested so much time and effort, it becomes difficult to stop and admit defeat! 'Banding EMR of this junctional lesion is not going to plan! Biopsies had diagnosed IMca at least but both EUS and CT had been reassuring. WHAT WOULD YOU CONSIDER NEXT?
■ Inject below lesion and try again
It will not work here and (rarely works at all)
■ Change to a wider cap
Will not work!
■ Remove lesion by ESD
You are asking for trouble!
■ Bail and declare that lesion is beyond endoscopic cure
There is no shame in admitting when you are beaten!
explanation
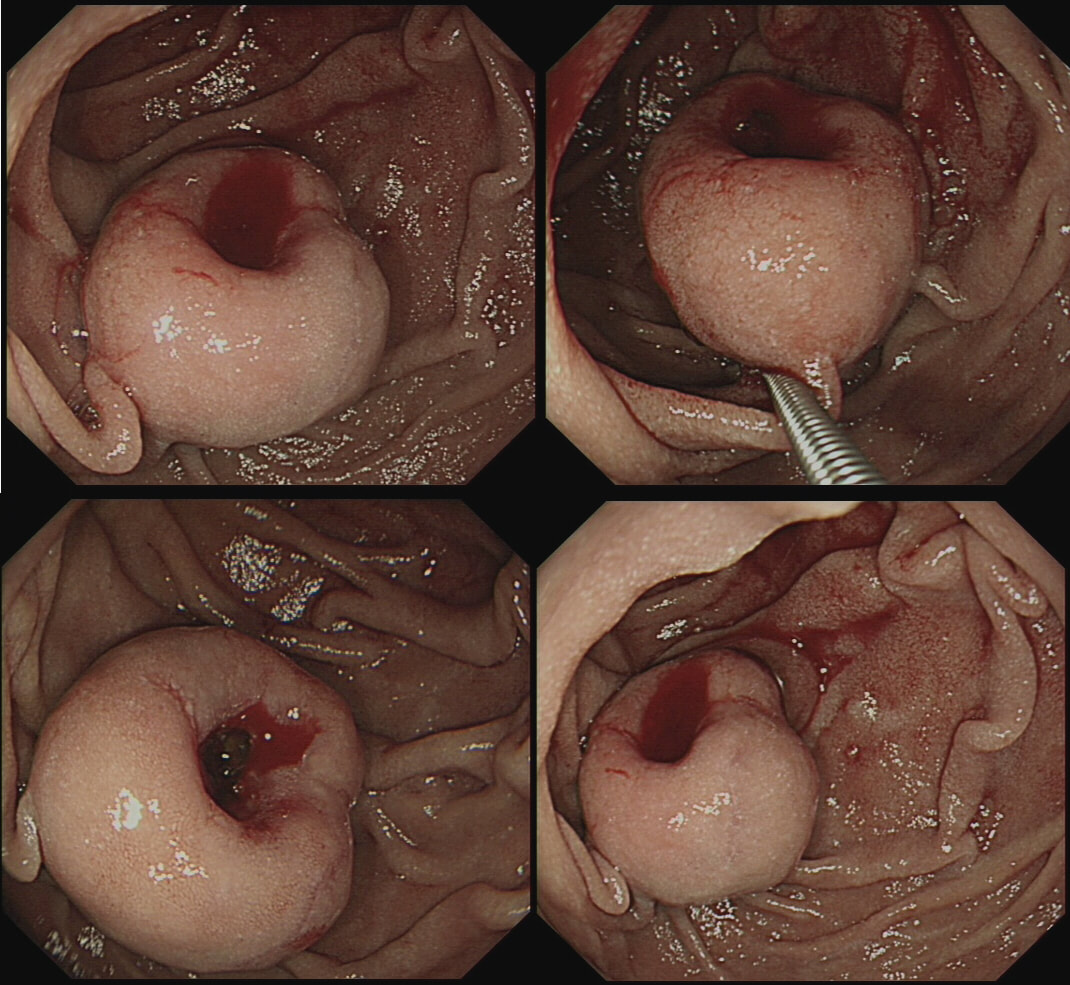
Although EUS and CT had both been reassuring, this lesion is clearly tethered down to the oesophageal wall. The lesion is extending too deeply for endoscopic cure. Trying to 'push the boat out' by attempting removal by 'pull within the snare' technique or ESD will fail and will run the risk of perforation, potentially resulting in the upstaging of the lesion. Bail out ! During ESD, the endoscopists encounter these vessels in the rectum WHAT WOULD YOU DO?
■ Back off, these are too large!
Don't be a chicken!
■ Apply Coag with the knife
They are too large for that!
■ Apply a couple of clips
They would get in your way!
■ 'Cook' with forceps
Of course!
explanation
This duodenal lesion was an incidental finding in a 55 year old patient with dyspepsia.
WHAT IS THE LIKELY DIAGNOSIS?
■ GIST
Could be but something else is more likely
■ Leiomyoma
These are rare in the duodenum!
■ NET
Yes, most duodenal NET's are umbilicated!
■ Adenoma
Duodenal adenomas are often IIc lesion but not like this!
■ Carcinoma
Too pretty to be malignant!
explanation
Umbilicated lesions in the duodenum are usually NET's. Even when the NET is as small as 7-8mm they will usually start to develop a central dimple as in the example below !
Ampullary NET's have a far worse prognosis than NET's situated elsewhere in the duodenum and are therefore first considered for a surgical resection. However, a small non-functioning, non-ampullary NET's may be considered for endoscopic resection.
I don't think that I was thinking entirely clearly when I accepted the referral from the Neuroendocrine 'multi-disciplinary team' for an attempt at endoscopic resection. The lesion is about 2cm and I quoted a 1:10 risk of requiring an emergency surgery in case of a perforation. This was probably an underestimate because NET's develop in the submucosa and you almost have to scrape them off the muscle propria layer which is VERY thin in the duodenum. Furthermore, I think that a small unrecognised 'micro-perforation' in the stomach is probably often 'sorted out' by a timely intervention by our great friend - the omentum! The duodenum on the other hand is retro-peritoneal and bathing in pancreatic juices. After 12 hours, that 1mm perforation will be 1 inch across and the patient will be in a desperate state. For this reason, if you do undertake duodenal resections you should be backed up with a 24-hour/day 364 day/year specialised pancreatic emergency surgery service. It's no good having an breast surgeon trying to patch things up! Now being an older and more experienced endoscopist, I believe that a far more sensible cut-off for endoscopic resection of a duodenal NET is probably 10-15mm. Personally, in an old person with comorbidities, who are unlikely to survive an emergency laparotomy, I would decline if I thought that the risk of emergency surgery was <1:100. Of course the NET could grow but is unlikely to turn malignant and kill an 85 year old patient with a previous myocardial infarction and dodgy kidney function. Particularly if the lesion is 'WHO grade is 1' only ! Of course Rembackens Resection Rule #15 applies: "A good endoscopist knows when to resect and a great endoscopist when to reassure! Also known as "Disengage if there is Disease".
Large polyp at the recto-sigmoid junction on a short stalk which I have pre-injected.
WHICH DIATHERMY SETTING WOULD BE MOST APPROPRIATE?
■ Pure cut, effect level 1
it will bleed like stink !
■ A blended cut, effect level 4
Absolutely, steady does it!
■ Coagulation, effect level 1
Too much heat and too little cutting!
■ Coagulation, effect level 4
WAY too much heat and faaar too little cutting!!!
explanation
Endoscopy is an "art" and there is certainly more than one way of resecting polyps. A large polyp (turned out to be a TVA+LGD) could only be sustained by a plentiful supply of blood and therefore immediate bleeding is the most important concern. Removing it piecemeal could reduce the risk of bleeding but would be inappropriate as there is a risk of cancer, nestling deep inside the polyp head.
As you know, I am not a fan of placing clips beforehand. This is polyp is a good illustration why. A few clips on the stalk would get in the way, may lead the current to the nearby colonic wall and are unlikely to compress the vessels DEEP inside that stalk.
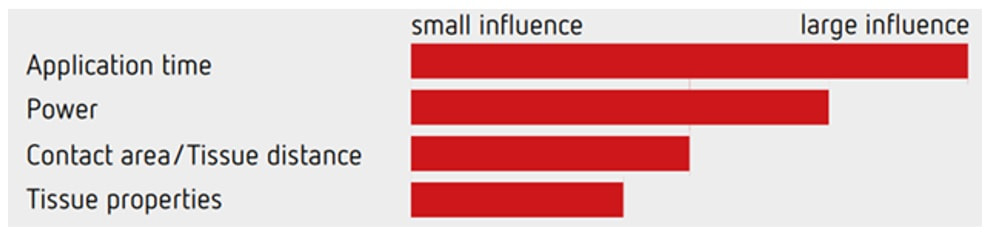
The graph above illustrate the four factors which influence the speed of your cut! The most important is the speed of closing the snare, the second is the power setting of your diathermy, the third is the thickness of your snare (thicker, braided snares take longer to cut through a polyp and will impart more heat) and finally the tissue properties (fatty tissue is slower to cut and I guess that a fat patient will require more electricity for the same about of cutting power).
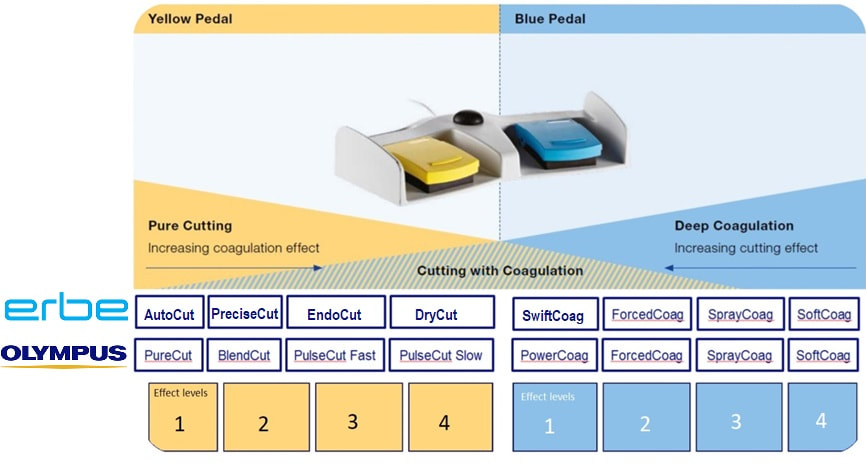
Of course you can decide on the speed of snare closure but closing that snare yourself, or asking your assistant to for example count to 10. By the way, a good tip is to ask your assistant to stop applying pressure on the snare handle when the snare is visibly starting to cut through the stalk. In my experience the 'inertia' in the system will continue the snare closure at the correct speed whilst continuing to close the snare handle often cuts the centre too quickly. Anyway, this thick stalk could never by cut through with the blue pedal coagulation current. It's simply too thick and you would end up imparting an enormous amount of heat to the tissues. You may consider 'pre-coagulating' the stalk by first applying some coagulation power and then finish with the yellow pedal. It sounds clever but I suspect that the last bit which gets 'cooked' is the centre which carries the vessels. Therefore I hardly ever do it anymore. A 'pure cut' (called 'AutoCut' by ERBE and 'PureCut' by Olympus would be inappropriate as it would cut the stalk too quickly leading to immediate bleeding. You would only use pure cut setting for sphincterotomy, cutting a short oesophageal stricture etc. This leaves you with the correct answer which is B !
Six months ago a SSL+HGD had been removed from transverse colon. This is the EMR scar.
IS THERE A LOCAL RECURRENCE?
■ No, site looks OK
No it so doesn't !!!
■ Yes, looks like a local recurrence
In fact, it's a malignant local recurrence !
explanation
You will be surprised to hear that samples taken was reported as 'normal mucosa'!!! Fortunately, someone didn't believe this and organised an early follow up for further samples. This confirmed adenocarcinoma with LVI !!!
Bizarre how a lesion can seemingly turn cancerous after resection! Presumably what happened is that it was cancer all along but this wasn't recognised at the 'index histology'. The moral of the story? Don't take the histological diagnosis as the 'bottom line'. These guys are experts but at the mercy of accurate targeting of samples and they only have a few tiny scraps to look at. You on the other hand, can see the whole lesion. If histology, doesn't seem to correspond with your endoscopic resection, more tissue is needed. On the topic of endoscopic assessment, ALWAYS look at lesions in both anteversion and retroversion !
This polyp was removed from the ascending colon. Histology confirmed that the lesion was a TA+LGD. However, it also mentions an underlying lipoma ...
WHICH STATEMENT DO YOU AGREE WITH?
■ There was no lipoma!
That's right!
■ Hmm, may have missed that?
You didn't miss anything!
■ Yes, this was noted at the time
The submucosa is often 'fatty' in the right hemicolon!
explanation
It's common for the submucosa on the right side of the colon to be laden with fat. In fact, you can see the fat globules in the bottom-right image where the lesion has been turned up-side-down. It can be a little alarming to see fat floating about in the mucosal defect after the resection . You may think that you have perforated but it's normal !
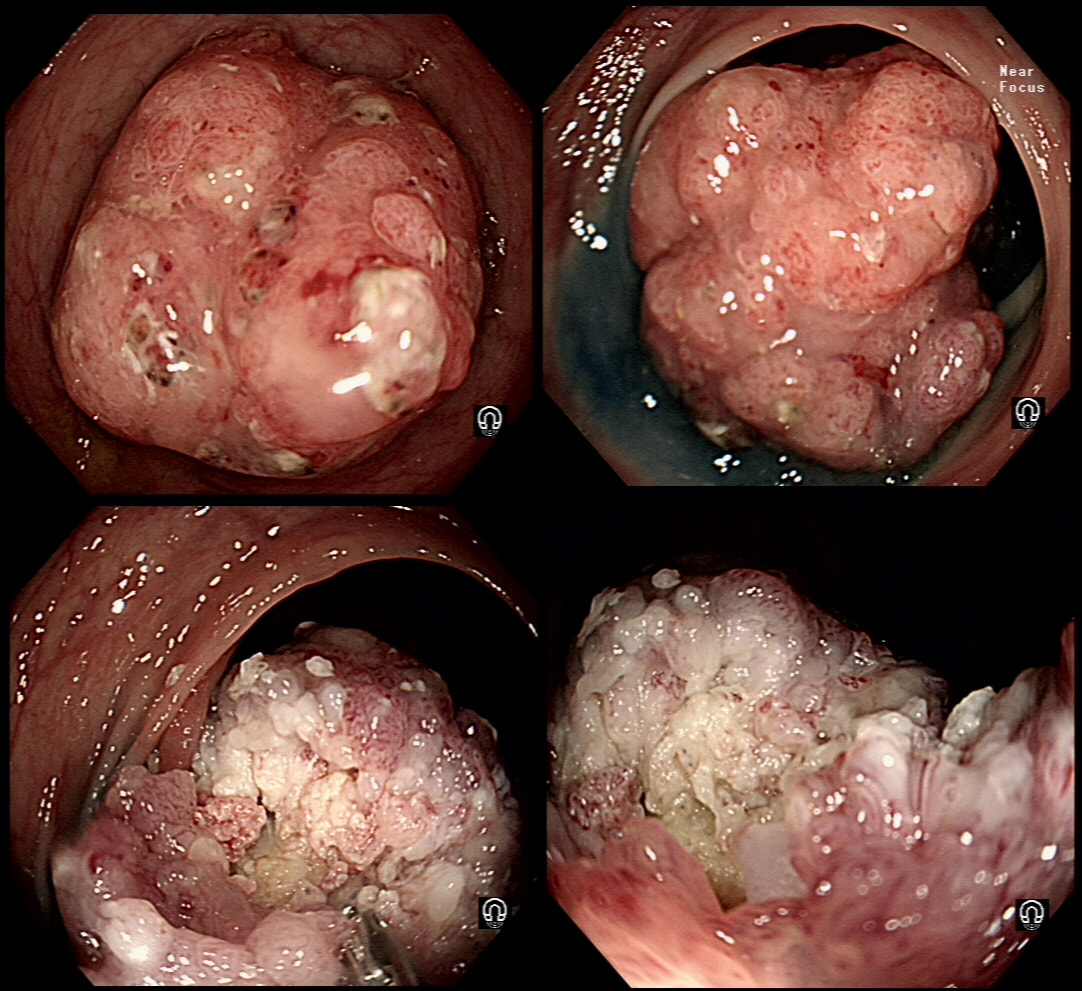
This 70 year old gentleman presented with PR bleeding and mucus. WHAT IS THE DIAGNOSIS?
■ Rectal prolapse
Nope, doesn't look like this!
■ Active proctitis
Seriously? How long have you been scoping?
■ Solitary rectal ulcer syndrome
SRUS can look almost like anything but not like this!
■ Rectal adenoma
Yes, to be specific it's a villous adenoma!
■ Rectal carcinoma
That polyp is big but not ugly!
explanation
Perhaps something of a 'noddy' question as any endoscopists worth his salt should recognise this as a large villous adenoma. However, many endoscopists would not see beyond the sheer size of the lesion and conclude that the lesion must be malignant SOMEWHERE! The truth is that it's precisely because the lesion is inherently innocent that it has grown to this size WITHOUT turning malignant! So how to deal with it? An endoscopic resection would include a circumferential resection for about 12cm, starting at the anal verge. There are several options; ■ Perhaps you could remove it in sections? For example, removing a quarter and then bring the patient back after a few month to do another section? I've tried it! Sadly, they grow back so quickly that by the time the patient returns, it has already re-grown to recover the previously cleared surface ! ■ Perhaps you could use APC, not to clear the lesion but to reduce the amount of blood-stained mucus it produces? I've tried that too!!! Unfortunately, APC tends to make the mucus production worse !!! ■ There is one more thing which I have learnt with large villous adenomas like this. They tend not to lift very well! As the lift is always 'shallow', you end up with a very narrow submucosal window to dissect through. Similarly, a piecemeal EMR is almost impossible unless the VA is much smaller. ■ The only thing which we haven't tried in Leeds is a joint effort whereby 2-3 endoscopists take it in turns to dissect the lesion over a 8-10 hour period, perhaps under GA. A daunting prospect and I doubt that it's actually feasible. Naturally, a TEMS procedure is similarly unlikely to succeed and the patient is probably most likely to wake up without a rectum at all. However, loosing the rectum to benign disease at the age of 75 would be a bitter pill. Perhaps that's why most choose to put up with the mucus ?
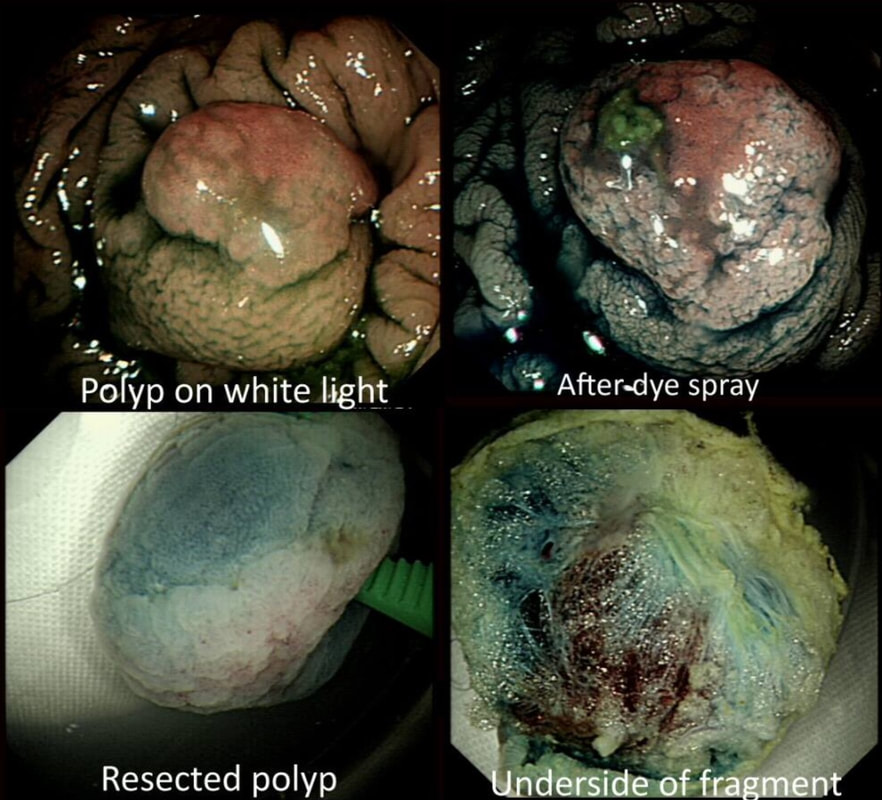
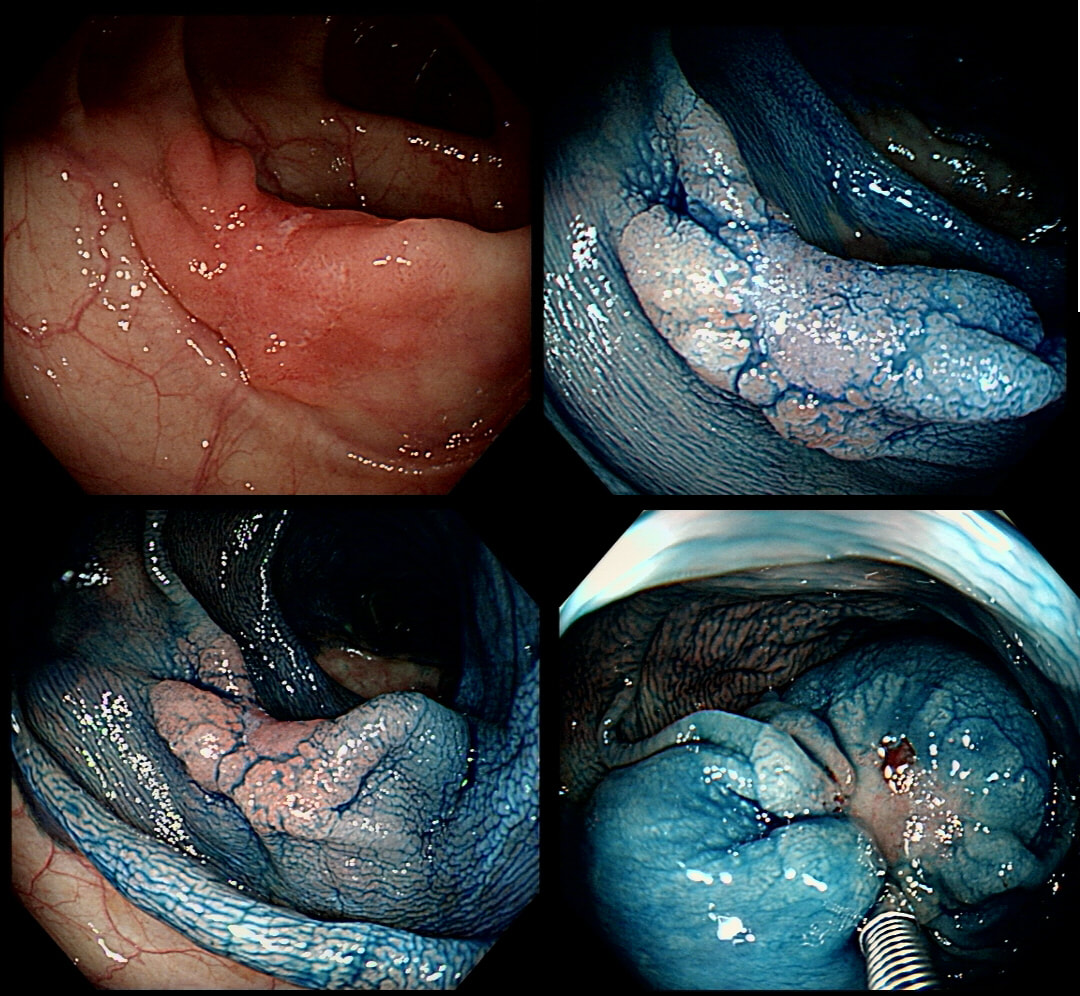
The lesion above is removed 'full thickness' and turns out to be an sigmoid carcinoma extending up to, but not involving the muscle propria layer (i.e. it's a T1 cancer with sm3 invasion). Histology reports clear margins but when the patient returns for a 'site-check' endoscopy, the nodule below in photograph B is found at the resection site ...
The mysterious nodule is removed and sent for analysis (Histology below as photograph C ) .
WHAT IS THE DIAGNOSIS?
■ Granulomatous/inflammatory reaction
Doesn't look like it!
■ Mucosal prolapse reaction
Yes, what else could it be!
■ Local recurrence of adenoma
Doesn't look adenomatous!
■ Local recurrence of carcinoma
Could be a concern, perhaps submucosal deposit but histology reported clear margins ...
Explanation
Endoscopically, this doesn't look like anything! It doesn't look either inflammatory or neoplastic (i.e. adenoma or cancer). Why then was it removed?! Endoscopically, this looked like normal mucosa but what then is it? To find out, the lesion was removed. I had my suspicions as to what was going on and took great care to close that tiny, unremarkable looking resection site with 4 clips.
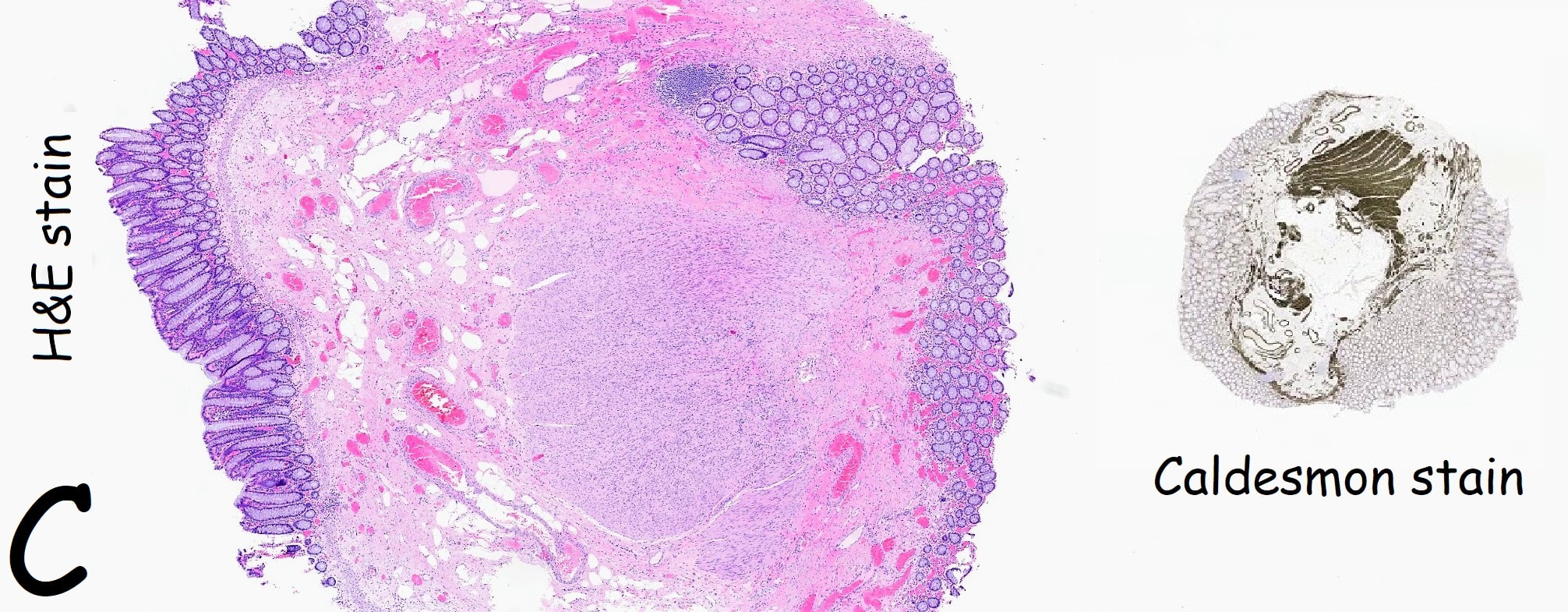
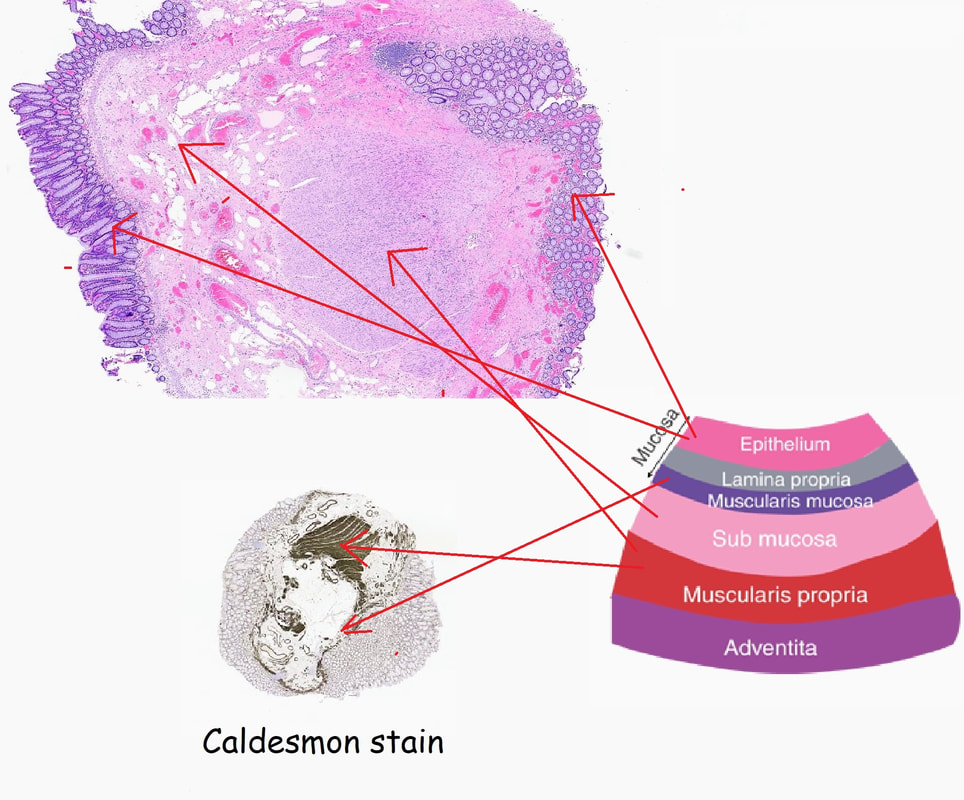
Histology did confirm that this is a full thickness mucosal prolapse at the FTR site. Of course, the point is that if you don't recognise this and resect it, you will end up with a delayed perforation !!! I've attached an annotated histology slide below. As a professional you should understand histology. By the way, the 'Caldesmon stain', shows up muscle. That large grey blob is of muscle propria in the centre of the 'polyp' and the thin grey layer around the periphery, just below the surface epithelium (the polyp is cut in a horizontal manner), is 'muscularis mucosa'.
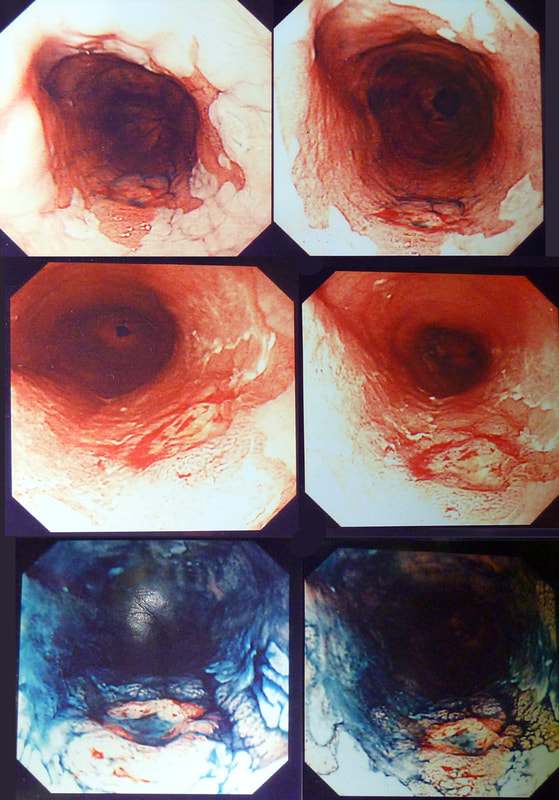
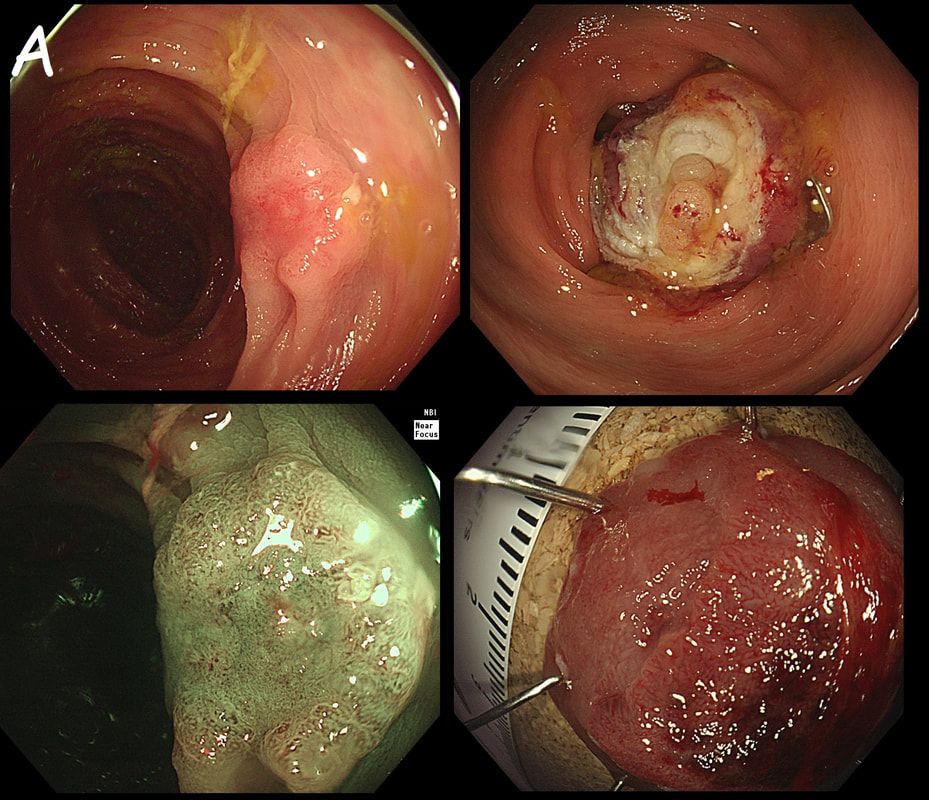
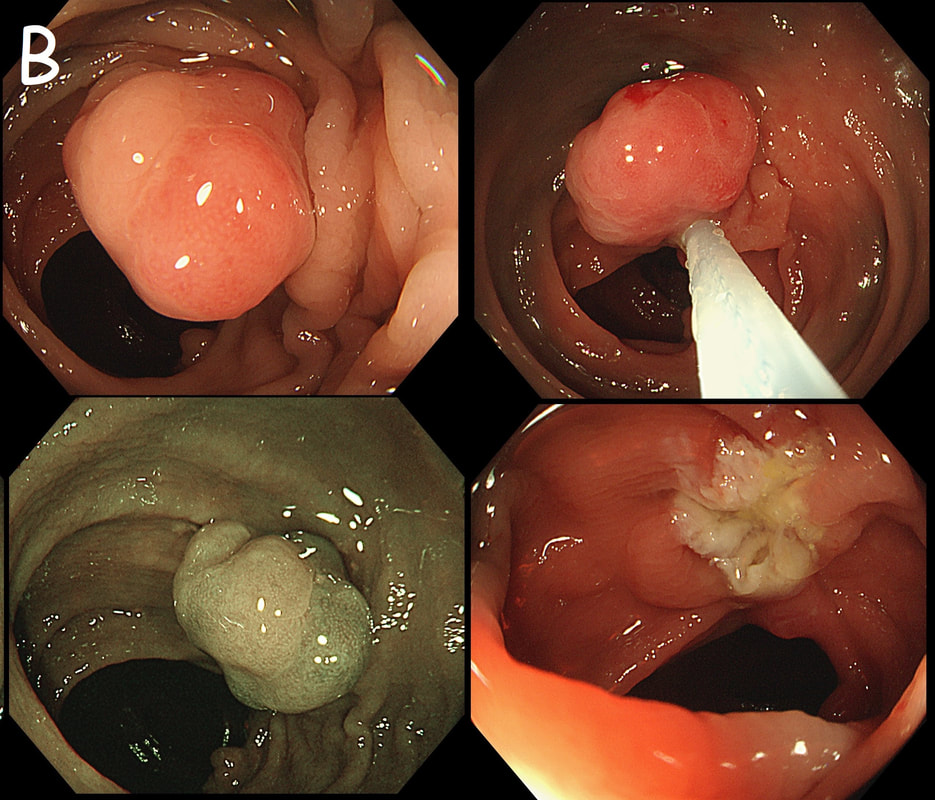
Flat polyp found in the ascending colon of an elderly patient with anaemia
WHAT DO YOU THINK TO THE LIFT?
■ Good - Attack!
Hmm, you are an optimist here!
■ Borderline - proceed with caution !
Yes, or bail out unless you are sure of what you are doing !
■ Poor - Abort !
There is no shame in bringing this back to the Cancer group to think again!
explanation
Histology confirmed that the centre was a poorly differentiated cancer which was 'probably completely removed but only with a <100 micron clear deep margin. As I keep saying, don't expect there to be "entirely good news" after an endoscopic resection of a colonic cancer.
So what happened? In spite of the original surgical misgivings the 80 year old patient subsequently underwent a right hemicolectomy which did confirm that the lesion had been completely removed by the earlier ESD. Would a full thickness resection had made any difference? Of course not! The centre of that cancer would still have been poorly differentiated!!!
Is it only me who still still sometime spray "maalox" (the antacid) onto mucosal defects to find the precise origin of that annoying venous oozing ?
WHAT IS THE NAME OF THE 'SIGN'?
■ Rasberry & Cream
Nearly but nobody eats rasberry & cream!
■ Strawberry & Cream
Oh yes! You are clearly as old as I am?
■ Rasberry & Milk
Nope!
■ Strawberry & Milk
Less fattening but wrong!
explanation
Of course this is the "Strawberry and Cream" sign from 20 years ago ! Great for spotting where that pesky oozing is coming from in mucosal defects or perhaps in peptic ulcer bleeds (although I have never used it in that setting for some reason). The disadvantage is that once you have covered that mucosal defect with maalox, you can't see much else! Therefore, make sure that you have removed all of the lesion and you are happy that there is no micro-perforation before you consider using maalox.
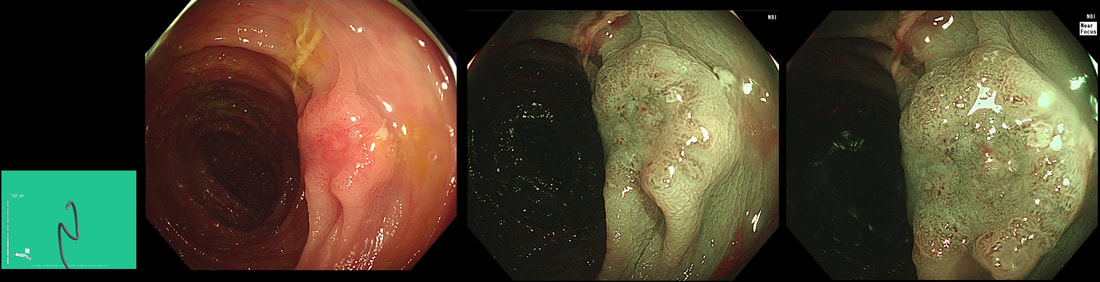
This was found at the caecal pole in an 75 yr old man. Two years ago, a small adenoma had been removed from this spot.
WHAT WOULD YOU DO NEXT?
■ Ignore the lesion
Perhaps reasonable if patient is unlikely to live more than a few years
■ Keep under surveillance
Reassuring to everyone but may seem ridicilous if patient is turned down for a resection at the age of 80, following 3 heart attacks and a stroke ...
■ Attempt another EMR
Very unlikely to succeed if original resection was done by someone with experience
■ Attempt a full thickness resection
Don't you need further information before deciding?
■ Refer surgically
Uncertain as not studies have compared endoscopic vs surgical approaches
explanation
The crux of the problem is two-fold: First, what is the risk that this lesion will turn cancerous and kill the patient and second what would be the risks of its' removal. • It's a little below 10mm in diameter and according to the data from the NHS BCSP, the risk of cancer within the lesion would be 1.5%. You could perhaps argue that it may be a little greater because it's a local recurrence. On the other hand the surface biopsies had been reassuring only showing a TA+LGD and this probably reduces the risk closer to 1%. The risk of the polyp turning cancerous in the future in the main depends on how long the patient lives. A Norwegian study by Eide T et.al. (Int J Cancer 1986;38;173–6) put the annual conversion rate to cancer for an ‘average’ colonic polyp at 1.25% at 5 years, 2.5% at 10 years and 5% at 20 years. A barium enema study (Stryker S. et.al. Gastroenterology 1987;93:1009–13) of polyps larger than 1cm, estimated the risk of cancer to be 2.5% at 5 years, 8% at 10 yrs and 24% at 20 years. There is also a CTC study of 306 polyps in which only 6% of polyps in 6-9mm range grew larger than 10mm at an average follow up of 2.3 yrs (Pickhart PJ et.al. Lancet Oncol. 2013;14(8):711–20). My own rule of thumb is that if the patient has a life-expectancy below 10 yrs, there may be little to gain by prophylactically removing lesions. This also fits with the latest UK postpolypectomy surveillance guideline which suggest that it should only be offered in patients with life-expectancies greater than 10 yrs (Rutter M et.al. Gut 2020;69:201-23). There are life expectancy calculators available online (just click the link). • As regards 'risks of removal', at one end of the spectrum you have the risks of an 'average colonic polypectomy' (for a subcentimetre polyp. I usually quote a ≤1:200 risk of late bleeding and ≤1:1000 risk of a perforation). Not all polyps are the same and at the other end of the risk spectrum, you have the risk linked with an 'ampullectomy/papillectomy'; 1:10 risk of acute pancreatitis for up to a few months after the procedure, 1:10-20 risk of late bleeding for up to 2 weeks after the procedure, 1:50 risk of a perforation, a 1:10 risk of late stenosis, 1:20 risk of acute cholangitis and a 1:200 risk of death ... • So what can a full thickness resection offer? A recent retrospective review by Ichkhanain Y et.al. from 18 centres (12 in the USA, 5 in Europe and 1 Canadian centre) reported on 66, young fit patients with smallish lesions (avg size 15mm). Surprisingly, 26 of the 66 lesions did not extend deep into the appendix lumen and arguably could have been removed by conventional EMR !? The authors don't elaborate and probably didn't have any further information than that 14/66 lesions had failed a prior attempt at conventional resection. 90% of patients were given prophylactic antibiotics and everyone was admitted after the procedure. Most (70%) of procedures were done under propofol anaesthesia but that is probably the normal approach in the US. The average procedure time was 1 hour. The overall chances of a completely successful endoscopic clearance was 75% (50/66). As regards complications, 17% developed appendicitis (10/58) and 6 pts these patients required surgery. I presume that the appendicitis was rather severe in these cases as patients staid in hospital for an average of 5 days following their surgery! The prophylactic antibiotics did not seem to make any difference to the risk of appendicitis. The success rate of 75% doesn't compare all that well to the reported success rates of ESD which was 95% but that study was small with only 34 cases (Jacob H et.al. Endoscopy 2016; 48: 829-36). • What can your surgeons offer? Insertion of a straight stapling device across the caecal pole whilst pulling on the appendix would probably be a quick surgical procedure without any risk of post procedural appendicitis and a 100% chance of complete clearance. Of course it would be more expensive and the usual hazards associated with a GA and surgery would be in play. By the way, Michael Bourke at Westmead Hospital in Australia writing in Gut 2021;70(2) points out that the surface area of the appendix constitute 7% of the caecal surface area. You will be familiar with a similarly sized triangle of mucosa between the two ends of the ileo-caecal valve and the appendix - the so called 'lawyers triangle' (for obvious reasons)? Michael highlights a study of 34 patients with the 'Serrated Polyposis Syndrome, serrated lesions was found in 23 and some were dysplastic (Pathology 2016;48:30–4). Unfortunately, the single most important fact is missing - what proportion of patients with 'Serrate Polyposis Syndrome' actually develop cancer of the appendix? To summarise, I don't think that it's possible to give a 'correct answer' for this question. The 'correct decision', would depend on the patients life-expectancy, the histology of the polyp (HGD would push you towards resection of course) and whether there is anything to suggest that the patient would not 'do well' if he/she developed severe appendicitis requiring emergency surgery afterwards.
This 3cm polyp was removed from the sigmoid colon. After returning to the recovery area the patient is complaining of severe low abdominal pain.
WHAT WOULD YOU DO NOW?
■ Keep in 'recovery area' for observation
Not 'wrong' a such but you can do more!
■ Bring back into endoscopy room to apply more clips
Now is your chanse to do this before that peritonitis takes hold!
■ Organise a CT
In my experience, it often muddies the water and adds little useful information in this scenario
■ Admit, organise a CT and start AB's
Not 'wrong' but placing clips is more important than any of this
explanation
Large sessile colonic polyps are a particular challenge for endoscopists. That surface crypt pattern may not be an accurate reflection of what is hiding inside! When there is cancer hidden at the centre of the polyp, this will induce a fibrotic response (the pathologists call this a 'desmoplastic response') which can tether the polyp to the muscle propria layer. Large polyps being yanked about by peristalsis can also develop fibrosis beneath even if they are benign (example below of an entirely benign VA+TSA harbouring LGD only with dense fibrosis in its centre). Of course, non-lifting is usually obvious when the lesion is small or flat but can be impossible to see below a large sessile polyp.
The first sign that something is amiss is when in spite of 20-30 seconds of yellow pedal power (i.e. a blended cut/coag/pause diathermy cycle), the polyp is still in place! When this happened, I used to curse under my breath, re-set the diathermy and with a strong hand and a further 30 seconds of diathermy, I would usually 'win' the battle and the polyp would fall off. Unfortunately, this would often turn out to be a 'Pyrrhic victory'. There would either be quite alarming bleeding or a perforation. Nowadays, I believe that these large, sessile polyps are best removed by ESD. In a slow, controlled manner you can then dissect below the lesion. If you encounter fibrous tissue you can attempt to dissect around it, abandon or (if you've got the balls) attempt to continue the dissection in the same plane through the fibrous tissue. Of course, you can also deal with those chunky vessels, one-by-one in a more controlled manner. Back to the scenario in question. If I think that there could be any risk that I've perforated, I would bring the patient back into the procedure room to place LOTS OF CLIPS. In truth, I did immediately recognise the peri-colonic fat in the middle image on the right and closed the perforation without delay. That white base to the upturned polyp in the last image, is a sizeable chunk of muscle propria layer. Anyway, provided that there is no peritoneal contamination, the perforation has been thoroughly closed with a virtual 'suture line' of clips and the patient is young and 'sensible', I would observe them for another few hours in recovery and discharge provided that they remain free of abdominal pain. Beware of the patient who remains in pain but claims to be comfortable because he is desperate to get back home... Of course, the patient must understand my verbal and written information that there may have been a small perforation which I have closed with clips but that he/she must return to hospital if further pain develops. Of course, my standard post-resection information leaflet always advice patients to return to hospital if bleeding or pain develops up to 2 weeks after the resection. No antibiotics, no scans, no admission!?! Controversial of course and I must stress that this is my own management strategy. I believe that my way of dealing with micro-perforations is safer than admission (exposing the patient to all the in-hospital hazards of multi-resistant bugs, Covid-19 etc), reduces the risk of 'over-treatment' (CT scans after EMR's or ESDs' always look alarming to the radiologists with gas in the wall of the bowel and the peritoneal cavity which means that surgeons will be reaching for their knives) and cheaper (of course). By the way, I am no great fan of the over-the-scope (OVESCO) clips for three reasons; a) time is of the essence in perforations and with the OVESCO clip you loose time but having to withdraw the scope to attach the device and b) it is very cumbersome and it can be difficult to reach the lesion and c) you only get one chance to get the clip in the correct position. With 'normal clips, it doesn't matter if a clip goes on a little wonkily, you just place another one!
This 13mm colonic lesion has been referred for resection
WHAT IS YOUR PREDICTED HISTOLOGY?
■ Benign adenoma
No way! This looks like cancer!
■ SM1 cancer
This was our own diagnosis although the non-lifting was of a more advanced lesion!
■ SM3 cancer
Doesn't really look like it but this was the CORRECT answer!
■ T2 cancer
Wrong because the lesion should then have NO crypt pattern
explanation
Histology had 'informed us' that this was a TA+HGD. But of course, Endoscopically this IS A CANCER! 'Newbies to endoscopy' may wonder how I can be sure?
Simply, this is what early colorectal cancer looks like !!! Of course, some cancers are hidden deep within larger, sessile or pedunculated polyp and can not be glimpsed from any disturbance of the polyp surface. That is a separate issue and no amount of experience can help you with the chance finding of cancer buried deep within such a polyp. Attempting to find the words to describe the features which are 'malignant' is difficult. Firstly the lesion is firm! If you can reach it with a finger, you will find that it's hard to the touch. If you can poke it with the biopsy forceps you will find that it moves like a solid disc of firm tissue. It's also the elevated margin surrounding an angrily red centre which has a different crypt pattern is another feature. Actually, there does appear to be some sort of crypt pattern in the centre. Endoscopically we had diagnosed sm1 invasion (i.e. invading into the top 1mm of the submucosal layer). Surprisingly, it didn't lift at all and because the patient was not a surgical candidate, we offered a 'full thickness resection' (images below). Rather surprisingly, the final diagnosis was of sm3 invasion with LVI! With time you will find that removing cancers endoscopically never leaves you with absolute 'peace of mind'. There is always some adverse feature to keep you awake at night! This polyp was found in the transverse colon. It has not previously been sampled! WHAT IS THE LIKELY HISTOLOGY?
■ SSL
INCORRECT as the crypts are slit-like
■ Adenoma with LGD
INCORRECT as the central dip says otherwise
■ Adenoma with HGD
CORRECT!
■ Superficial cancer
INCORRECT because the lesion does lift
explanation
This is a rather interesting polyp. Well its starts off looking rather booring but after the submucosal injection it changes. Rather than a bland looking TA, it appears to be somewhat tethered in it's centre. I suspect that the subtle central dip would have been evident on retroflexion but retroflexion in the ascending proved impossible. Just look at how the scopeguide shows the shaft keeps buckling in the sigmoid and transverse, in spite of abdominal pressure to try to limit its movement. With suboptimal lift, you of course have three options: ESD, piecemeal EMR or choosing a more powerful, superstiff (but more dangerous) snare. I opted for the latter option (I usually do) and with some difficulty managed to capture the polyp single fragment. Arguably, as this lesion is a 'LST-D' type of lesion a piecemeal resection may have been less appropriate. The LST-D's are usually either HGD/IMca or superficially invasive cancer. The lift is good, telling you that it's not an invasive cancer after all. . On the other hand, a single fragment ESD may be linked with a 1:20 risk of a microperforation. In all honesty, probably not any different to the risk of perforation with a superstiff snare! This lesion was found in the descending colon. It's lifted with our standard EMR solution (volpex, adrenaline and indigo carmine dye). HOW WOULD YOU NOW BEST APPROACH THE RESECTION? a) the lift is poor, the lesion appears tethered and I would refer for surgery b) this is a benign polyp and a piecemeal resection using a 15mm snare would be best c) the polyp may be malignant and a large, floppy snare would be best to remove the lesion in as few pieces as possible d) preferable for 'single fragment resection' but a stiff, large snare would be preferable explanation
The lifting is a little 'lob sided' but that is common and not necessarily a 'showstopper'. A polyp of around 3-4cm would have a 1:7 risk or so of harbouring cancer. For this reason, removing the lesion either single fragment or in as few pieces as possible, would be preferable.
It's worth highlighting that when you have a sessile polyp like this, the surface crypt pattern may not accurately reflect what is happening deeper inside the polyp. So how could this polyp be removed in as few pieces as possible? Of course and ESD is the obvious answer. But it's not possible or appropriate to offer this to every patient. Another possibility would be to do a 'pre-cut' EMR, when you cut a grove around the polyp before resecting it. An 'underwater resection' would be a third possibility However, the simplest way to remove this as a central main fragment and then some small side-fragments would be with a stiff snare. A stiff snare allows you to push down hard on the polyp and get it off in as few pieces as possible. Of course there is a far higher than average risk of perforating with a stiff, large snare. Use it with caution and be prepared for trouble. A pre-cut around the polyp could also help in achieving a single fragment resection. Ultimately, this lesion was resected as a main central fragment and several small side-fragments (image below). The ultimate histology was of a tubulovillous adenoma (TVA) harbouring low grade dysplasia (LGD). |
Categories
All
|