|
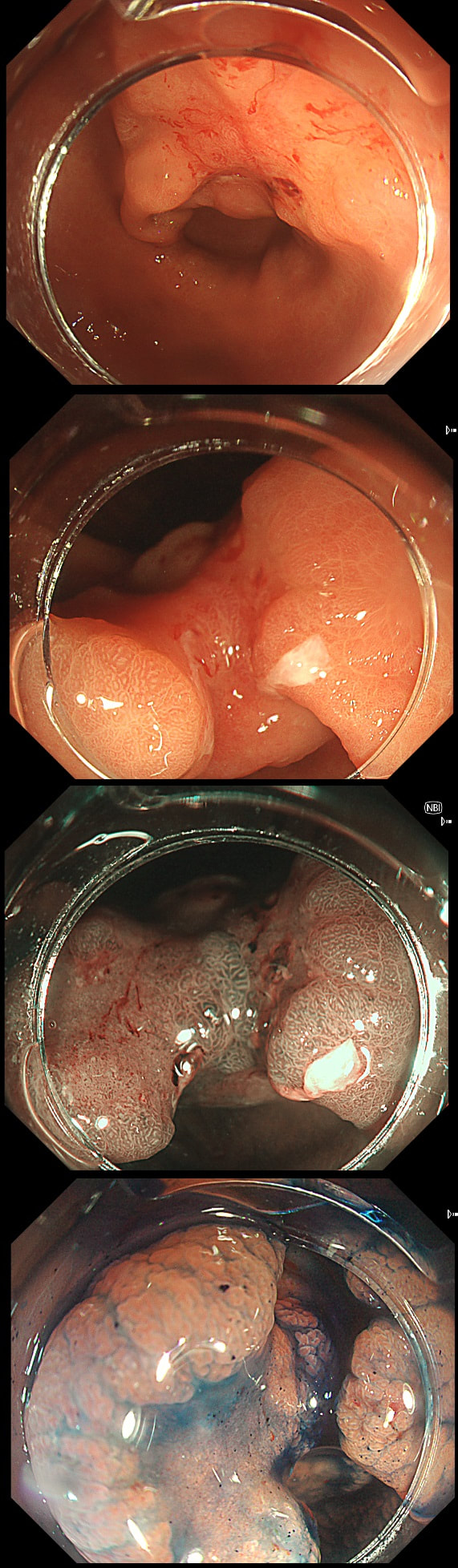
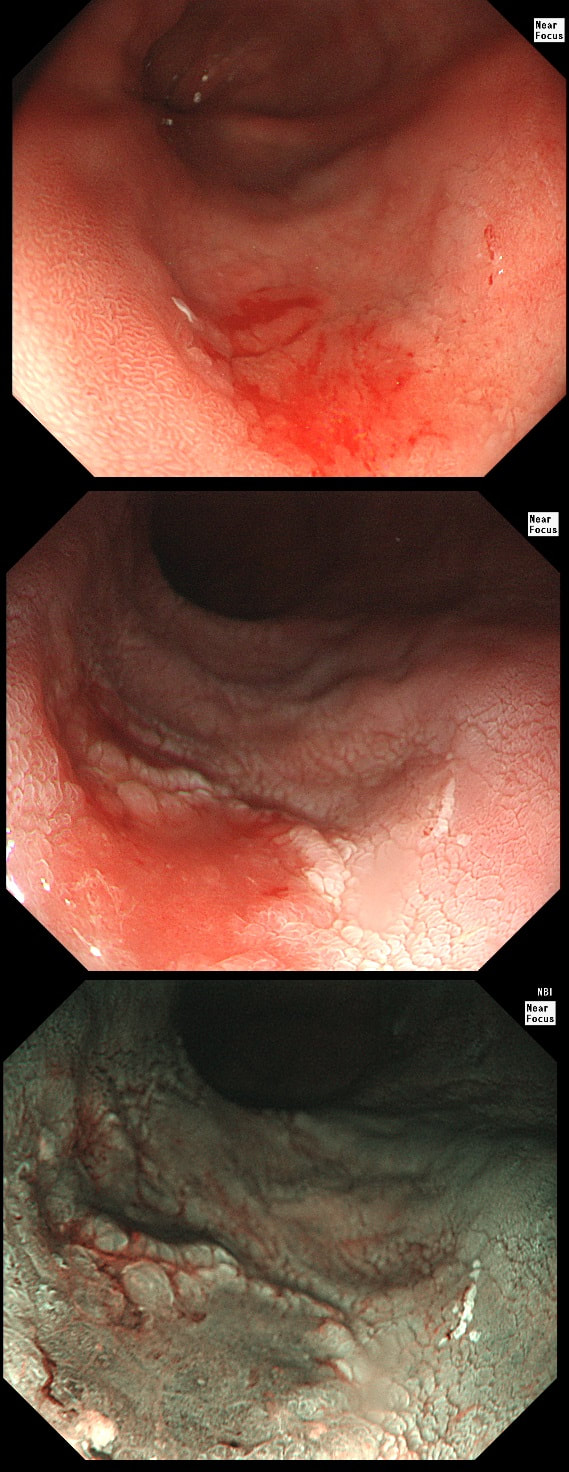
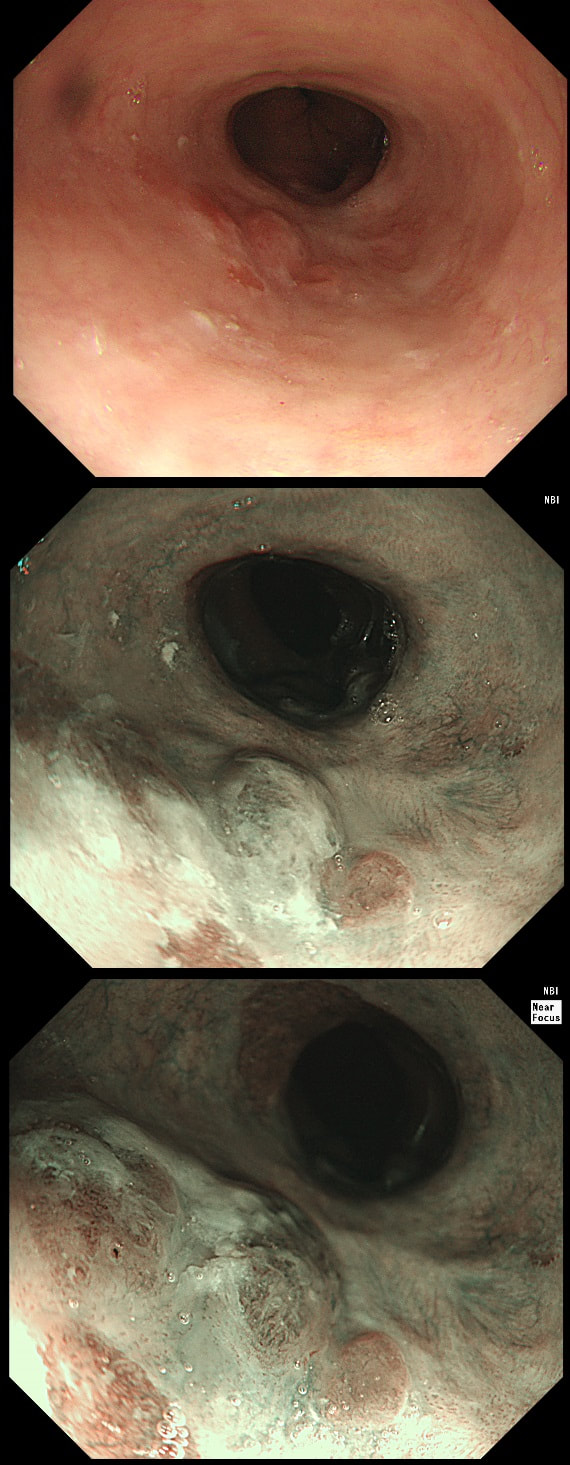
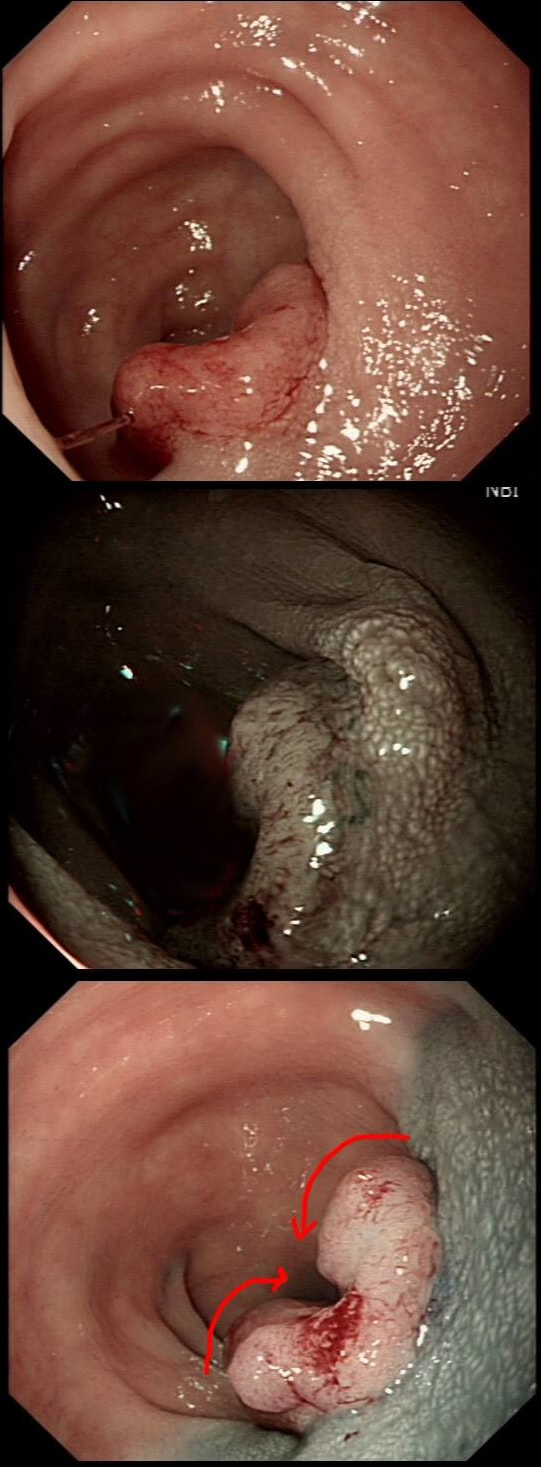
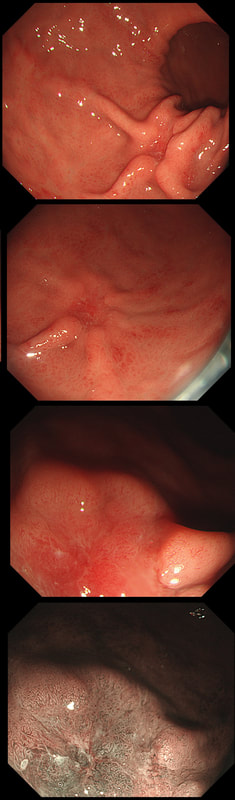
This gastric lesion is found in an 85 yr old man. Samples have confirmed a moderately differentiated gastric cancer but the lesion can't be seen on CT. The patient is frail with multiple comorbidities and declines both an endoscopic resection and surgery. However, he does ask how long it will take before he dies from the cancer?
WHAT WILL YOU ANSWER?
a) 1-2 yrs
Definitely too pessimistic!
b) 2-3 yrs
Hmm, perhaps if the lesion is poorly differentiated but it doesn't look it!
c) 3-4 yrs
This is probably how long it will before that cancer is at an advanced stage but patient will probably survive for another couple of years beyond that 'transition point'
d) 4-5 yrs
Probably too pessimistic still!
e) >5 yrs
With limited information this is the only figure backed up by some data.
Explanation
This question is something of an homage to Sir David Cox who developed the well known 'Cox Regression analysis'. Professor Cox died in 2022. As you know, the 'Cox Regression analysis' basically explores the relationship between the length of survival and more than one variable.
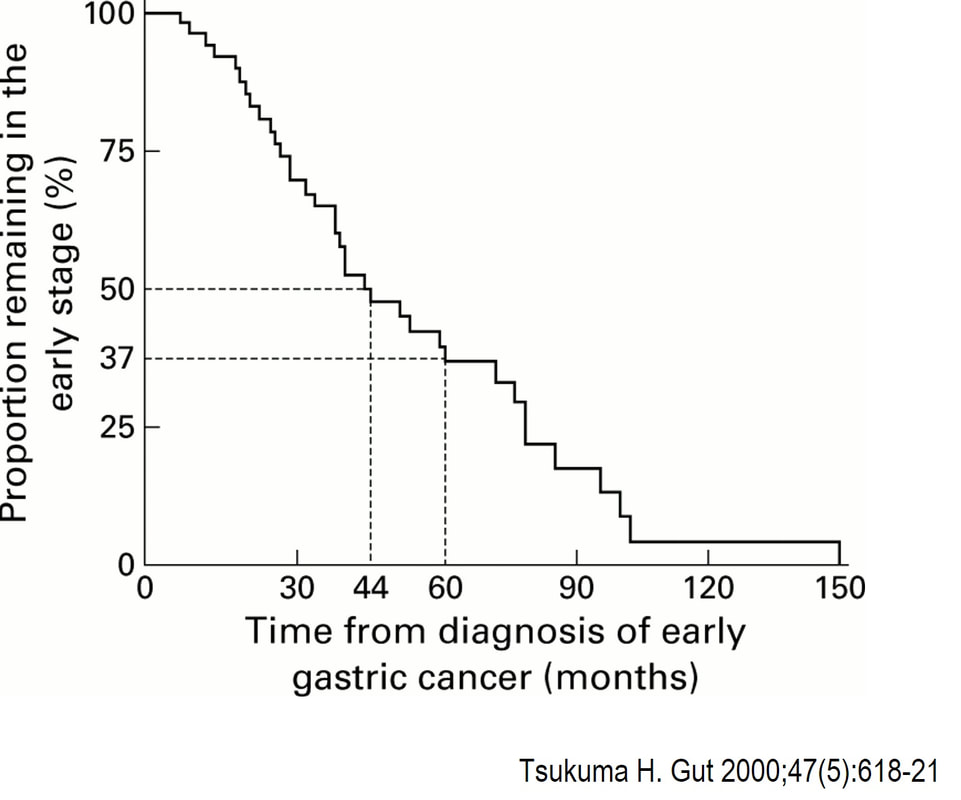
You would expect the following 'variables' to influence cancer-specific survival; histology, size of lesion, stage of lesion and perhaps the macroscopical type of early gastric cancer. Perhaps the location within the stomach also influences survival. There is some data that lesions at the gastric cardia are worse. Of course we don't have data on any of these 'variables'. However, I know of one paper by Tsukuma et al [Gut 2000;47(5):618-21] which did look at the natural history of 56 early gastric cancers in Osaka, Japan. This paper was actually a riposte to an earlier editorial from Leeds by one of my colleagues arguying that EGC is a 'pseudo-disease [Everett S. Lancet 1998;351:1350–2]. As you would expect, 56 EGC's is not enough for any sub-analysis of the above variables. Also they didn't actually look at survival. They looked at the average time it took lesion to progress from what appeared to be 'early' gastric cancer (i.e. confined to the mucosa or submucosa) as diagnosed by endoscopy. And here we reach the first issue! How sure can we be that the lesion in the image is an EGC and hasn't started to invade into the muscle propria (thus being advanced)? The lesion is about 2cm across and there is a an organised crypt pattern in the centre (can't comment on the vessel pattern as there are no magnification images). It does look 'early' and I stand at least an 80% chance of being correct in that diagnosis. Incidentally, 'lifting' is not a reliable sign in the stomach as any ulceration of the mucosa will tether it down, regardless of stage of invasion. The authors were pleased to report that if patients are left for long enough, those EGC's will grow and progress. No surprise there then! But the rate of progression was slow!!! The median time it took for 'early' gastric cancers to progress to advanced gastric cancers (i.e. T2 or worse) was estimated to be 44 months but probably shorter in patients with poorly differentiated cancers. Furthermore, the cumulative 5 year corrected survival in patients who never underwent surgery was about 63%. The answer to the question is therefore 'e'. This patient is likely to live more than 5 years from diagnosis of his early gastric cancer. Better than expected here is the Kaplan-Meyer curve below !
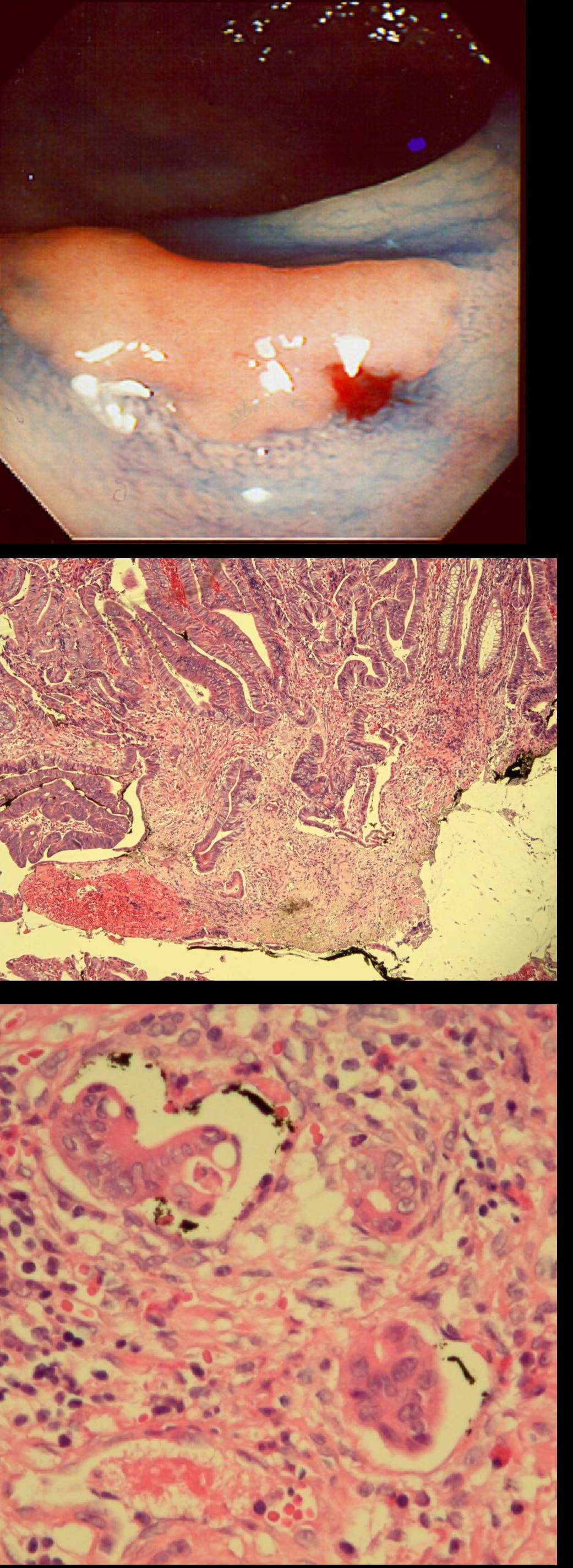
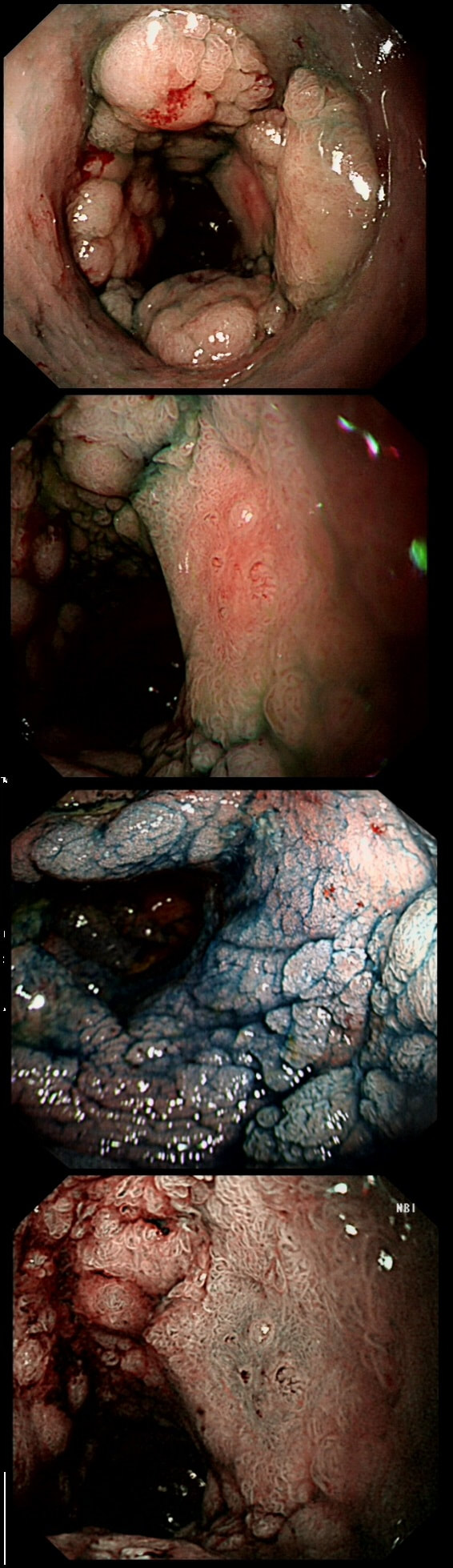
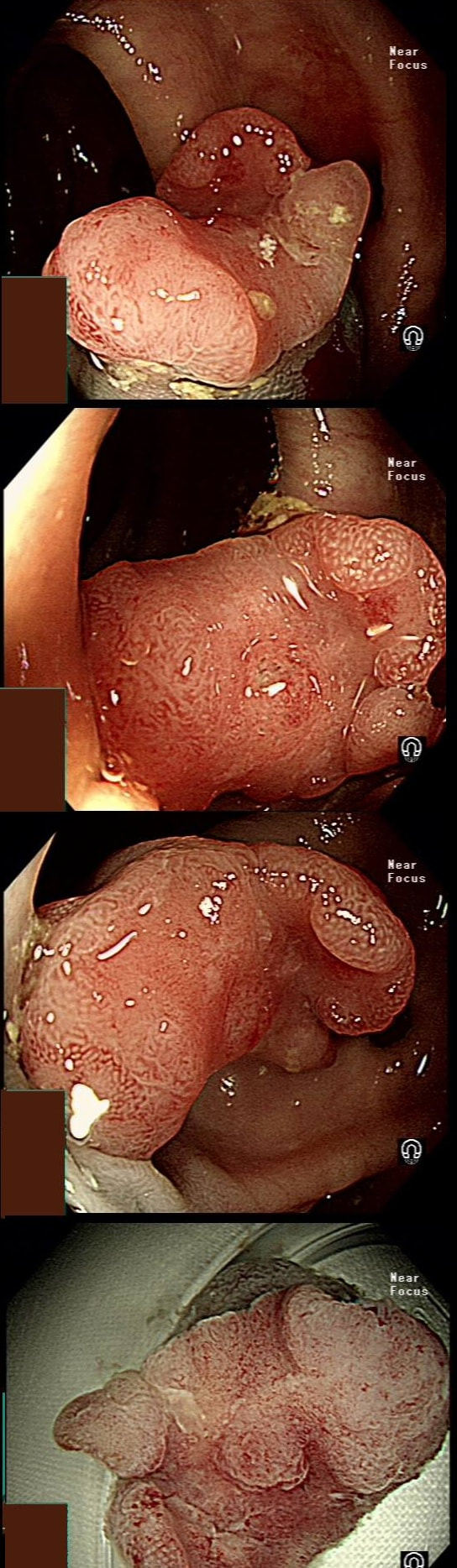
This patient underwent a CT angiogram for chest pain. The angiogram was unremarkable but the radiologist mentions an 'irregularity at the caecal pole with hyperenhancement and mild adjacent fat stranding' and recommend colonoscopy. After the examination, your patient asks you if you have found anything?
WHAT WILL YOU TELL YOUR PATIENT
a) Reassure the patient
That would be unwise!
b) Tell him to wait for the histology
Strictly speaking 'not wrong' but it's not the best option to choose
c) Tell him that we will organise an MRI scan next
MRI's are requested for rectal cancers
d) Tell him that we will organise an EUS next
Life is too short for EUS!
e) Tell him that we will organise a 'staging' chest/abdomen/pelvic CT
This is precisely what you should do!
explanation
Did you notice the lesion next to the biopsy forceps? At initial glance into the caecum, there is nothing to see. However, this lesion was about 20mm in diameter and with that rolled edge. It's clearly malignant and you need to request staging CT's. Histology did confirm a mucinous adenocarcinoma and CT sized it at 4.7cm. Far larger than initially thought! As it was involving the serosal surface, it was staged as T4, N1 (due to several small nearby nodes).
Mucinous colonic cancers are unusual, accounting for about a little more than 10% of CRC's. They are usually situated in the proximal colon. This is not the only reason why they are easy to miss. At the early stages they have an infiltrative, ulcerative growth pattern which easily hides behind bubbles or a pool. These are small but evil little things which are easy to miss and grow fast. By the way, 'signet ring adenocarcinoma' (where the mucus is INSIDE the cell rather than OUTSIDE of the cells) is another sub-type of adenocarcinoma which may be part of the same spectrum. Mucinous and signet ring adenocarcinoma, share similar molecular features such as MSI-H, CpG island methylator phenotype-high (CIMP-H), and frequent BRAF V600E mutations. Of course microsatellite instability is linked with Lynch syndrome but in this case immunohistochemistry stains revealed normal mismatch repair proteins MLH1, PMS2, MSH2 and MSH6.
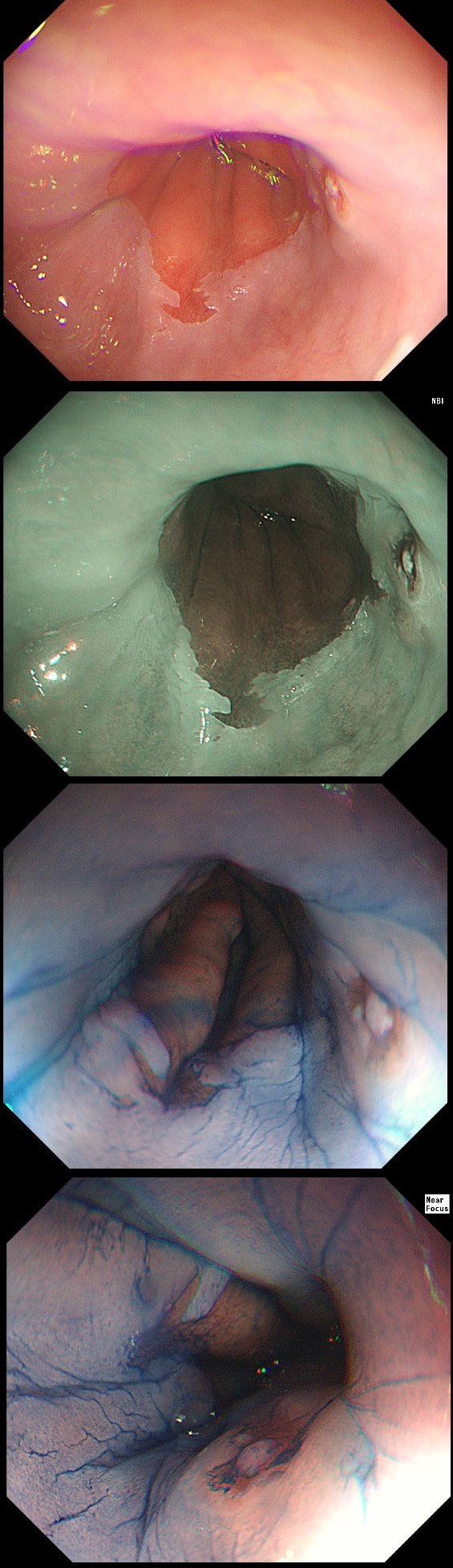
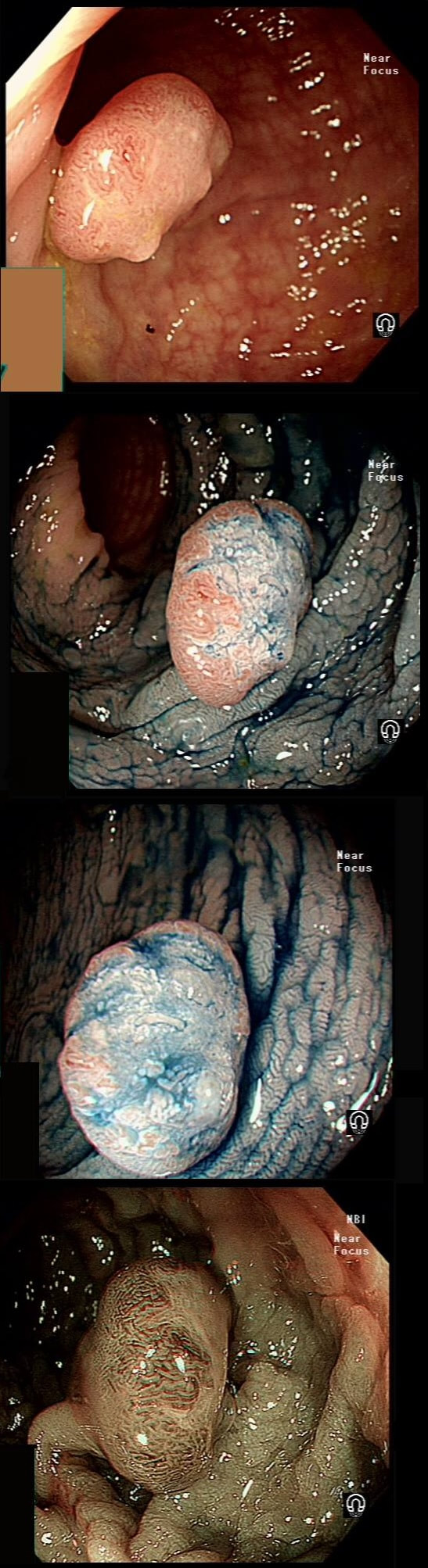
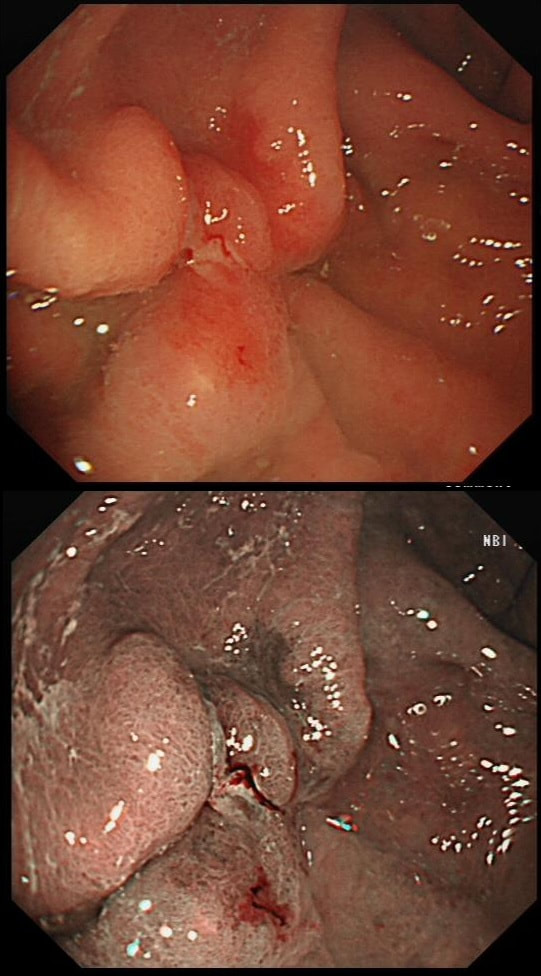
This 'lesion' was barely visible within in a Barrett's segment on white light. However, after acetic acid and with NBI it's more obvious.
WHAT IS THE LIKELY HISTOLOGY?
a) Barrett's LGD
Usually invisible or just a red, flat patch
b) HGD
Subtle change in crypt pattern but with little nodularity?
c) IMca
This was my own guess but it was wrong...
d) Invasive cancer
Yes, superficial invasion (100 microns) and poor differentiation!
explanation
I removed this lesion without worrying too much about the subtle 'depressed' growth pattern and the small, round crypts in the centre of the lesion. However, I was surprised to see the pathology report of a superficially invading adenocarcinoma, with poor differentiation to boot!!! This finding makes the advice on 'further treatment' more complex. As you know, in both the upper and lower GI tract, the finding of 'lymphovascular invasion' (LV) is probably the most 'ominous sign' that a patient needs surgery (or chemo-radiotherapy in case of the oesophagus). Poor differentiation is 'bad', but less bad than LVI.
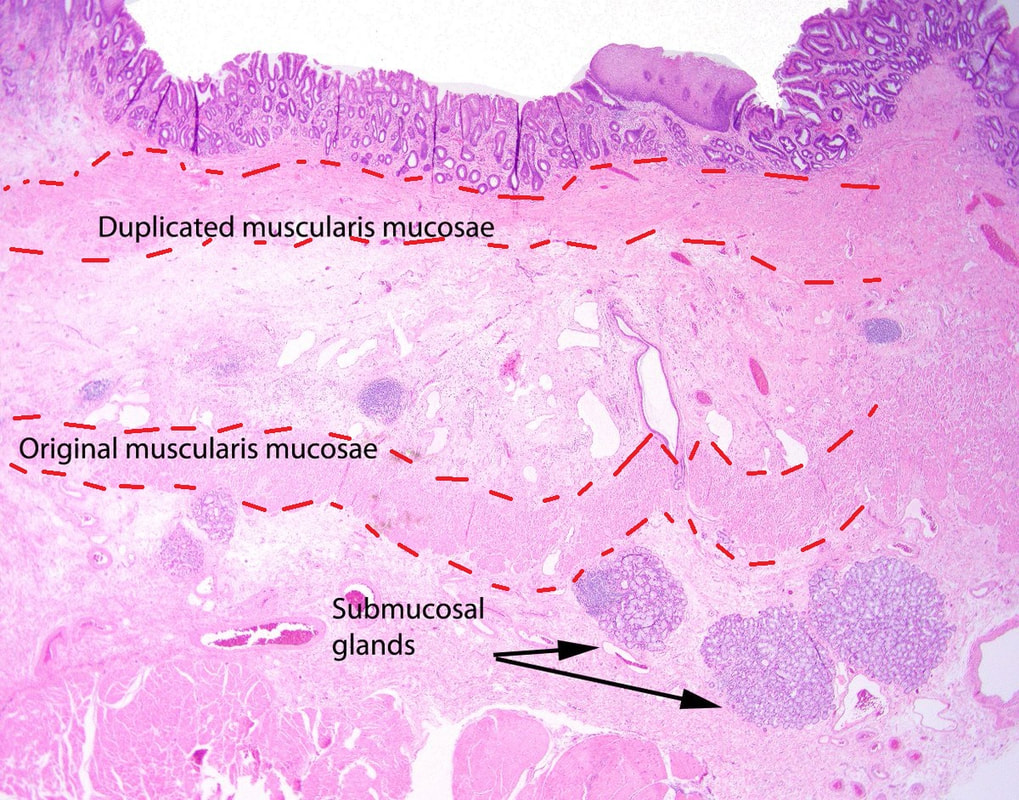
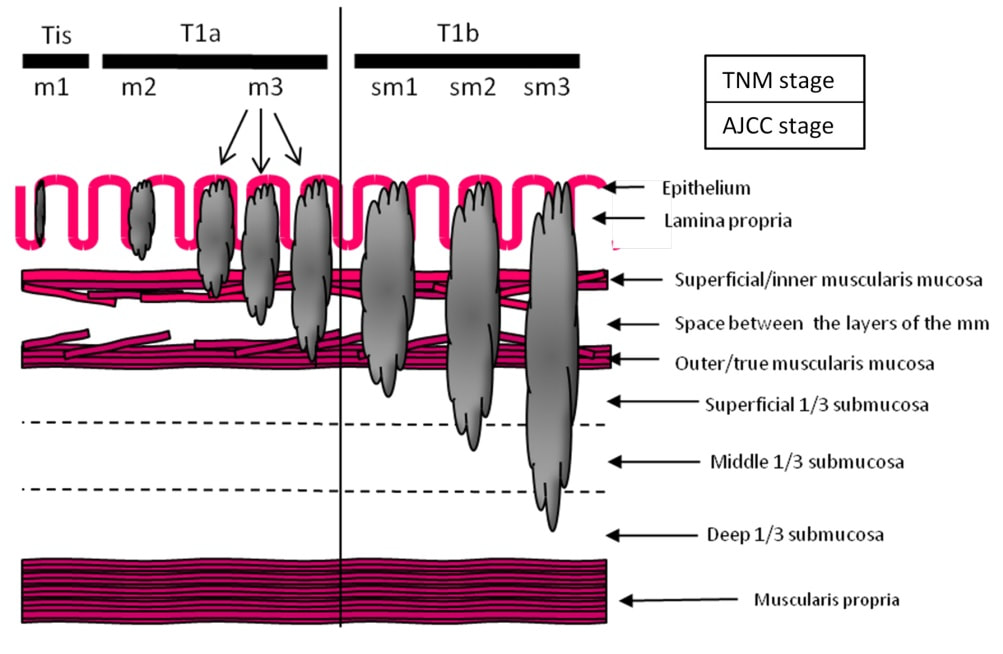
Depth of invasion is also important and in Barrett's you are 'allowed' invasion to about 500 microns below the muscularis mucosa. The corresponding 'safe margin' in SCC's is only 200microns. In this case the depth of invasion was only about 100 microns, leaving 'poor differentiation' as the only 'bad sign'. The patient wasn't a surgical candidate and refused CRT. This was 3 years ago and so far all is well! By the way, the histopathologists do have a more difficult job than you perhaps imagine, measuring the depth of invasion in Barrett's cancer. This is because they often see several bands of muscularis mucosa, so called 'duplication of the muscularis mucosa'. Elsewhere in the GI tract, the muscularis mucosa is a single band of smooth muscle. They measure the depth of invasion from the top of the muscularis mucosa down the the deepest point of invasion. However, if there are several bands of muscularis mucosa, which one do you measure from?!? Below is an example to illustrate the dilemma.
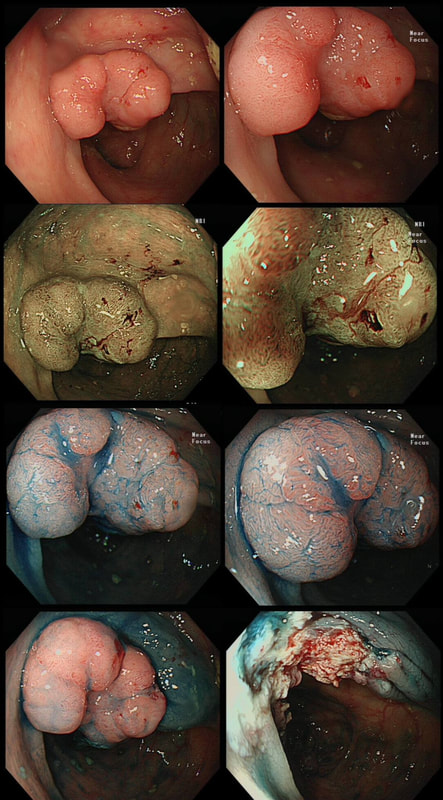
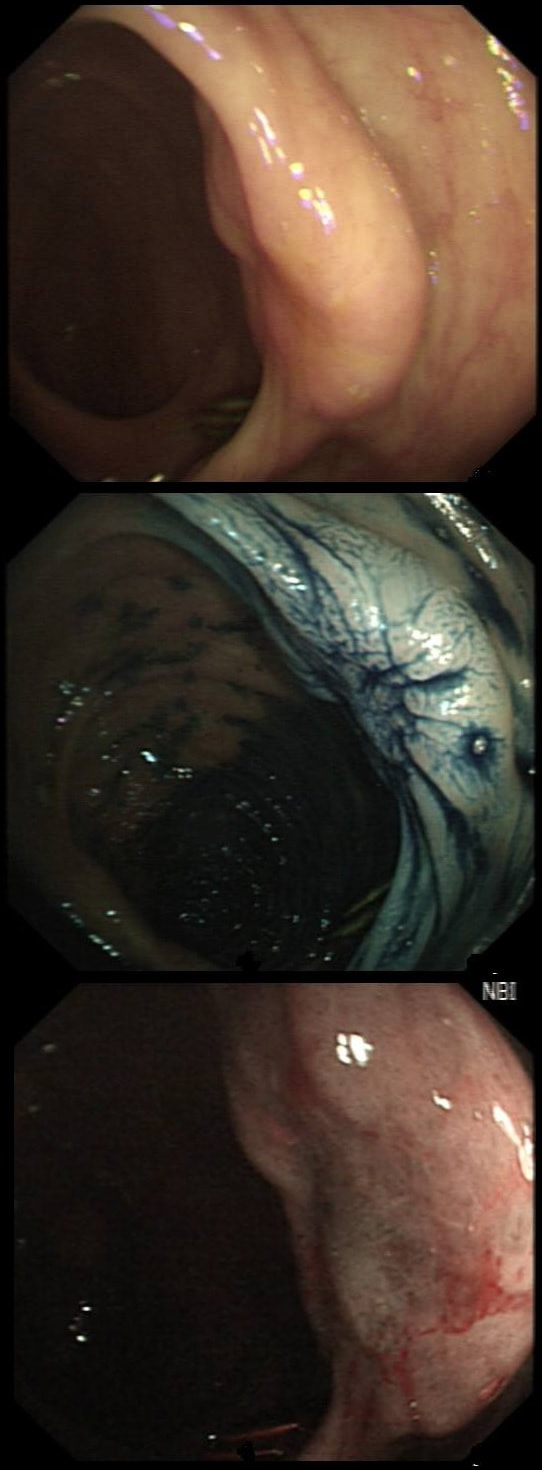
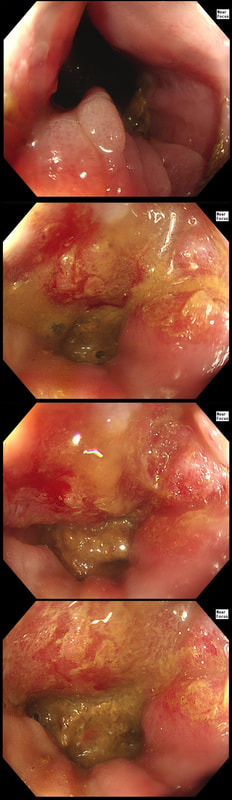
This lesion was found in the ascending colon and referred for removal.
WHAT IS THE LIKELY DIAGNOSIS?
a) HP
You must be joking!
b) SSL
You are not serious?
c) TA
Short slit-like crypts, well Yes but there is more to it!
d) TVA
Absolutely not!
e) Cancer
That non-lifting sign doesn't lie!
explanation
Must admit that I didn't like the look of this polyp. Sure, it does have an organised crypt pattern. I think that I can see short slits, making it a TA. This would fit with the fact that it probably is a LST-NG type of lesion (which are always TA's).
However, it also looks, 'chunky' and it's the 'thickness' of the lesion which made me suspicious. For this reason I was not surprised to find the non-lifting sign. As there is no lifting what-so-ever, I suspect that the lesion will turn out to be T2 at least.
Patient with Barrett's harbouring HGD has been treated with RFA. He has now returned for the second RFA session when this is found.
WHAT IS YOUR ENDOSCOPIC DIAGNOSIS?
a) Benign scarring
Doesn't look benign - Looks evil!
b) Local recurrence of non-dysplastic Barrett's
Barrett's are re-emerging in several places. Doesn't look benign!
c) Barrett's adenocarcinoma
Absolutely! Nodules are never allowed in Barrett's!
explanation
Actually, there is a re-emergence of several brown nodules below the squamous mucosa. Biopsies confirmed this as an invasive adenocarcinoma, re-emerging from below the 'neo-squamous mucosa'. Clearly, to try RFA again would be a mistake! The histology showed a 'poorly differentiated' cancer and we are recommending either surgery or chemo-radiotherapy next (CRT). Even if histology hadn't shown poor differentiation, this 'smells' like bad disease to me which we may well 'undertreat' endoscopically.
This patient attends for Barrett's surveillance WHAT IS YOUR DIAGNOSIS?
a) Barrett's without dysplasia
A nodule within Barrett's is NEVER 'normal'!
b) LGD
LGD is flat, not nodular!
c) HGD
Good guess! Although your guess actually proved to be wrong
d) IMca
Small crypts + nodule = correct!
e) invasive cancer
Nodule should have been larger and without those small crypt openings
explanation
My 'rules of thumb' when assessing Barrett's is as follows: 1) crypts look different in a distinct patch but the area is flat = LGD 2) areas of superficial ulcers within the Barrett's = widespread LGD 3) crypts look different and there is a distinct nodule = HGD 4) crypts are tiny small and there is a nodule = IMca 5) there are no crypts and there is larger nodule = invasive cancer Of course this isn't fool proof ! After all, it's very difficult to distinguish HGD from IMca even on histology! However, it gives you a starting point on how to assess Barrett's and what you should enter on that Histopathology request form. Remember that your pathologists need your help! This lesion was found at gastroscopy WHAT IS YOUR ENDOSCOPIC DIAGNOSIS?
a) Siewert I SCC
Nope!
b) Siewert I adenocarcinoma
Lesion isn't centred 1-5cm above the GOJ
c) Siewert II adenocarcinoma
Yes! Lesion staged as T2,N0 on PET-CT
d) Siewert III adenocarcinoma
Lesion isn't centred 2-5cm below the GOJ
explanation
This lesion is clearly malignant with a rolled edge and surrounding mucosal ulceration. It was confirmed as a Siewert II adenocarcinoma, T2,N0. Cancers at this location are becoming more common. They are easy to miss, particularly if you don't slow down as you traverse the gastro-oesophageal junction (GOJ) and/or retrovert at a distance. On retrovertion you need to pull the scope back up so that you can have a close view of the cardia. Siewert et.al. developed the classification as follows:
Of course it can be difficult to determine where the epicentre of a cancer is, particularly on imaging. At endoscopy you are in a unique position to accurately record the correct Siewert type of the cancer. It makes a difference because the Siewert type has implication for treatment! Siewert I lesions are treated with oesophagectomy and gastrectomy as these lesions usually metastasise to nodes in the mediastinum. Siewert II are 'true' junctional cancers and mainly metastasise to nodes in the abdominal nodes but in around 15-20% of cases, there are mediastinal nodes. For this reason, patients with Siewert II lesions are only offered gastroectomy (without oesophagectomy) IF there is no mediastinal lymphadenopathy. Arguably, any borderline mediastinal nodes should probably be sampled before or during surgery before a final decision is made not to clear the mediastinal nodes with the resection specimen. Patients with Siewert III cancers are usually offered total gastrectomy plus a distal esophagectomy (to get clear resection margins, a so called R0 resection ) as these lesions spread to peritoneal nodes. This is a video clip of a small lesion removed from the sigmoid colon. WHAT IS THE MOST LIKELY HISTOLOGY?
a) TA+LGD
But it's a IIc lesion with IIIs crypts in the centre?!?
b) TA+HGD
Absolutely!
c) Invasive cancer
But there ARE small round crypts in the centre and it DOES lift!!!
explanation
You may call this a "flat elevated lesion with a central depression (IIa+IIc lesion) or simply a depressed lesion (IIc lesion). Frankly it doesn't matter because both a part of the same 'family' of evil little b.....ds. They are always TA's and the small, round crypts (Kudo type IIIs crypt pattern) tells you that the lesion harbours HGD. This is because as dysplasia progresses from low to high grade, crypts get smaller and more withered. Of course they eventually disappear altogether as the lesion develops into a cancer which no longer follows any 'instructions' to form organised crypts. However, the crypt pattern is still discernible in the centre AND the lesion lifts well. Both of these tells you that the lesion is likely to still be benign. Ultimately, the pathologists called it a TA+HGD. However, there was mucinous differentiation in the centre of the lesion. Could these little shits be the early stage of mucinous colonic cancers? Quite likely! Imagine how easily they are missed when hiding behind a fold or below a shallow puddle !!! WHERE IS THE BARRETT'S CANCER?
a) 12 O'clock
Eagle eye!
b) 3 O'clock
But the round crypt openings say otherwise!
c) 6 O'clock
Nope!
d) 9 O'clock
Where there is hardly any Barrett's?!
explanation
Of course the lesion is situated at 12 O'clock. There you can see a subtle mucosal nodularity with an irregular vessel pattern. It was removed by 'suck within the cap' EMR and confirmed as an IMca. This lesion is being removed from the distal oesophagus WHAT IS THE LIKELY HISTOLOGY?
a) Hyperplastic polyp
You must be joking!
b) Adenomatous polyp
A gastric adenomatous polyp would surely now be malignant
c) Early malignant polyp
But it's arising from a malignant flat component!!!
d) Advanced gastric cancer
Absolutely!
explanation
Of course this is all very odd. Clearly this is an advanced cancer at the GOJ. What business do I have in 'attacking' this endoscopically?! Actually, the elderly patient had completed a course of chemoradiotherapy (CRT) for a T2, N0 junctional adenocarcinoma some 30 months previously. Now he has developed dysphagia and a CT confirmed a 2cm polyp at the gastro-oesophageal junction. Histology had shown 'at least IMca' and he was referred for consideration of an endoscopic resection. Clearly this lesion can't be cured endoscopically. In fact, the elderly patient is not a candidate for surgery and therefore there is no cure at all. However, I was thinking that as the cancer is mainly polypoidal, perhaps if the nodule could be removed, his swallowing will improve and he will not need a stent and could be offered brachytherapy. Clearly this was all 'speculative' but I'm glad to say that 6 months later, the patient still has not developed any dysphagia and his now starting brachytherapy. No doubt a better outcome than could be offered by a stent?
A polyp found in the descending colon and removed as a single fragment (H&E attached)
WHAT IS THE DIAGNOSIS
a) Tubular adenoma
Yes but you are only partly correct!
b) Tubulovillous adenoma
No way, this is a LST-NG!!!
c) Villous adenoma
Absolutely not - there are no villi!!!
d) Sessile serrated lesion
Looks like it but histology doesn't!
e) Malignant polyp
Full marks!
explanation
This is a LST-NG type of lesion (laterally spreading tumour of the non-granular type). They are always TA's (tubular adenomas) and often (but usually not), harbour HGD or cancer. I guess that we can't really be sure about the crypt pattern as this is a non-magnified image. However, looking at the histology slide with narrow crypts, I expect that the crypt pattern is probably IIIs (small round crypts) which goes with TA+HGD. Must admit that I was surprised to find invasive cancer and LVI (lymphovascular invasion) in a small lesion such as this! The last image shows clusters of malignant cells within lymphatics. Of all the 'markers' to suggest that the patient needs surgery, LVI is the most important!
Patient was complaining of indigestion and reflux symptoms.
WHAT IS YOUR DIAGNOSIS?
a) Normal oesophagus
Not quite normal is it?
b) Reflux oesophagitis
This doesn't look like reflux!
c) Adenocarcinoma
Actually the correct answer!
d) SCC
Red nodules, even if surrounding by squamous mucosa are adenocarcinomas (usually)
explanation
You may be surprised to hear that the small nodule at 3 O'clock turned out to be an IMca! It was removed endoscopically. Of course, it's the question then arises; "Should we offer RFA". The BSG recommends this for patients with Barrett's harbouring flat dysplasia. However, in this case there is only a tiny, tongue of Barrett's in the 6 O'clock position! Actually, we just gave this a quick blast of APC (at a fraction of the cost of RFA) and it was gone. However, the patient remains on annual surveillance!
This colonic polyp was removed as a single fragment from a 60 year old lady. You can see the mucosal defect in the last image. The patient asks you what will happen next?
WHAT WILL YOU REPLY?
a) Can't tell, we will have to wait for the histology
You can do better!
b) In all likelihood there will be a site-check in 4 month or so
You are missing something!
d) You will probably need an operation
You've spotted the desmoplastic reaction!
explanation
The polyp looks very suspicious but did seem to lift and I therefore decided to go ahead and removed it using a stiff, large snare. It took a little longer than expected for the snare to cut through. Of course, the mucosal defect should be blue. In this case it's yellow! The polyp was malignant, invading about 1mm into the submucosa and you are looking at the 'desmoplastic' reaction generated by the cancer.
Apart from the sometimes deceptive 'non-lifting sign', there are two further signs that a lesion may be malignant. First, it may look smaller and smaller as you inject below the lesion (see example below). Another sign is that your blue sub-mucosal injection appears to lift the lesion until you retrovert and have a look at the other side. If you then find that it hasn't actually 'crossed the mid-line', there is fibrosis below the lesion preventing the fluid to disperse evenly. I was not entirely surprised to learn that the patient declined surgery. After all, he was 86 years old! He lived another 7 years and never developed any sign of bowel cancer. By the way, there is a theoretical risk of tumour seeding if the lesion is perforated during resection. However, when the perforation is done with a red-hot tool such as a knife or snare, the risk of seeding is surprisingly low. I have perforated a handful of cancers but have never had a case of late disseminated peritoneal disease. My Japanese colleagues (off the record), agree that the risk is there (some have seen it) but is low. If you decide to sample a suspicious looking polyp, you shouldn't use the same forceps to sample another lesion. This is because if cancer cells become lodged in the biopsy forceps, which are then used to sample another location and them become stuck in the biopsy, the histopathologist will diagnose cancer in TWO locations when in fact, there is only a single cancer.
This patient with ulcerative colitis has developed a polyp in the transverse colon. The lesion has now been sent for an endoscopic resection.
WHAT WOULD YOU DO?
a) Abort!
Smart!
b) Attack!
You are creating a problem of your own making!
explanation
Some would say; "if you can remove the lesion in that colitic colon, then 'do it'! The problem is that nothing may appear "irresectable" giving plenty of time, determination and poor judgement.
Many studies looking at outcomes of polypectomy in UC, excluded polyps >1-2cm or flat polyps. Other studies have included polyps arising outside of the colitic field or only have a short follow up period of a few years. Actually, most are coming to believe that when dysplasia develops in the colitic colon, it's not a 'random' case of bad luck. It can be the result of a long process of progressive DNA damage! At some stage we will be able to have a look at the state of the stem cell DNA in patients with conditions such as Barrett's, Colitis and atrophic gastritis. I think that we are in for a surprise ! In addition, did you spot the small focus of invasive cancer in the 2-3 O'clock position? Surprisingly, this was only T1 disease!
A polyp found in the transverse colon
WHAT IS YOUR ENDOSCOPIC DIAGNOSIS?
a) Tubular adenoma
You are half correct!
b) Tubulovillous adenoma
On the left side, the crypt's are slit-like rather than gyrate
c) Villous adenoma
VA's are soft like sea anenome !
d) Serrated polyp
No, the crypts are not large, round openings!
e) Malignant polyp
Yes, there are no crypts on the right-hand side !
explanation
Did you spot that cancer has developed on the right side of this tubular adenoma (TA). On the left side, the crypts are slit-like whilst on the right hand side, the crypt pattern has been destroyed (Kudo type V crypt pattern). The Haggitt stage was I and I took particular care in removing the polyp with a large margin. The cancer was only 4mm in diameter. Perhaps the smallest adenocarcinoma of the colon you will ever see ?
This is the scar following the piecemeal removal of a sigmoid polyp some 6 months ago. It was a 15mm tubular adenoma harbouring high-grade dysplasia. Samples from the EMR scar has showed 'distorted glands' only.
WHAT WOULD BE THE CORRECT FOLLOW UP?
a) organise an immediate follow-up
WTF ! This not a normal scar! You absolutely need to organise more samples, and perhaps a CT !!!
b) organise a follow-up in 6 months time
Would have been a mistake !
c) organise a follow-up in 12 months time
Would have been a big mistake!!!
d) organise a follow-up in 3 years time
May have cost the patient his life!!!
explanation
The first EMR was piecemeal and histology could of course not confirm that the resection had been complete. Indeed the 'index histology' reads oddly mentioning "frequent mitotic figures" and "back to back glands". To a gastroenterologist these words does not sound particularly alarming.
However, the pathologist was trying to say "this looks like cancer but I can't actually make that diagnosis" !!! Indeed this doesn't look like a normal EMR scar! The whole area is indurated as if there is an infiltrative process below the mucosa. Histology was reassuring mentioning some distorted crypts only. Sadly, the endoscopist was content with the reassuring repeat histology and did not reflect on the worrying endoscopic appearances. He did NOT organise a second round of post-EMR samples and the patient returned 2 years later with an advanced cancer. The take home messages from this sad story?
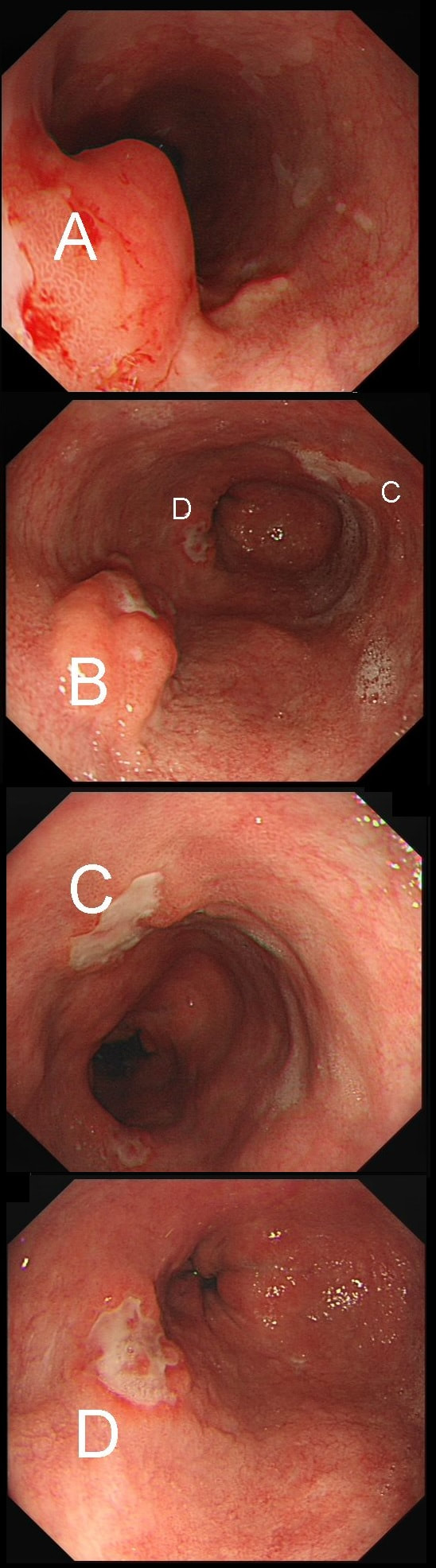
55 yr pt with a Barrett's nodule is referred for an endoscopic resection. I'm surprised to find 4 abnormalities within his 10cm stretch of Barrett's
WHICH OF THESE IS THE MOST LIKELY TO BE ENDOSCOPICALLY RESECTABLE?
a) Lesion A
I agree! Of these 4, A is the only one which isn't ulcerated and therefore is least likely to be invading deeply.
b) Lesion B
Would be my second guess as the ulcerated area seem superficial
c) Lesion C
Don't like the ulceration!
d) Lesion D
Would be my least favourite lesion to attack as the ulceration suggests deeper invasion and poor lift into my cap.
explanation
This may be something of a record, 4 synchronous lesions! Clearly A, B and D are malignant. At first, ulcer C seem more innocent without an elevated edge but on closer assessment, it also has a slightly elevated rim.
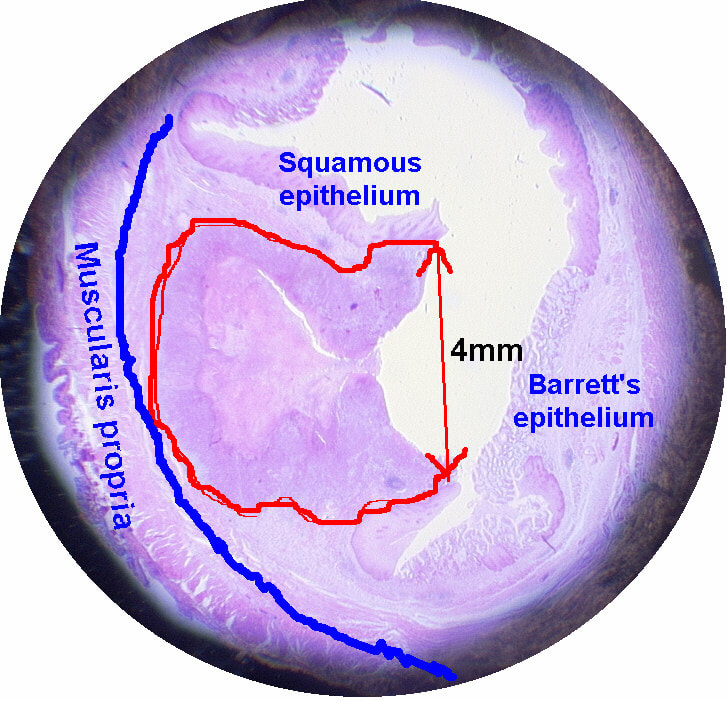
Of these 4, A is the only one which isn't ulcerated and therefore is least likely to be invading deeply. Of course, they are assessed together as there is no point in EMR'ing one only. Either all are curable endoscopic means or none are ! Rather than going ahead with attempting to resect these, I actually bailed out and took samples from each lesion. Biopsies showed invasive, poorly differentiated adenocarcinoma at each location! Clearly, this patient has multifocal 'bad disease' which endoscopy is unlikely to cure in my opinion. I believe that surgery is a far better option and the patient is currently awaiting his oesophagectomy. If you still are not convinced of the pitfalls in trying to deal with ulcerated Barrett's lesions, have a look at the lesion below. Two rounds of sampling had indicated that the lesion harboured HGD. However, I failed to remove the lesion and ultimately the patient underwent an 'Ivor-Lewis'. You can see the histology yourself. The 4mm surface is literally the tip of the iceberg and below you can see the cancer (red line) invading up to the muscle propria.
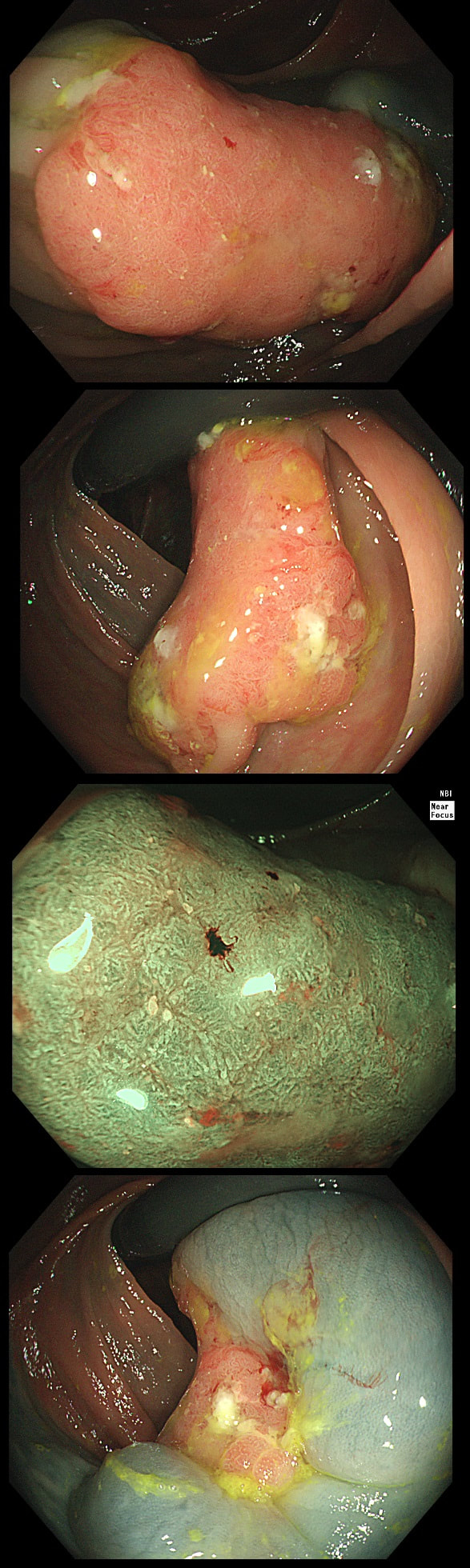
This polyp found on the lesser gastric curve.
WHAT IS THE MOST LIKELY DIAGNOSIS?
a) Hyperplastic polyp
HP polyps are usually subpedunculated and angry red
b) Intestinal metaplasia
IM is also 'pale' but not 'flat elevated'
c) Gastric adenoma
Would be my first guess!
d) Early invasive cancer
This is a plaque without a depression or dominant nodule to suggest invasive cancer
explanation
Of course, this could have been rather easy to miss! An insignificant pale, plaque-like lesion but with distinct borders best seen after indigo carmine dye spray (as usual). Endoscopically this is what a gastric adenoma looks like and it was removed endoscopically. Somewhat surprisingly, histology reported a small spot of IMca and therefore its at actually an early cancer - not invasive though!
Most invasive, gastric EGC's are of course shallow depressions as in the example below. In fact the lesion below was invading into the middle of submucosa and was confirmed as poorly differentiated. Afterwards the patient underwent a distal gastrectomy where no further cancer was found. 'Poor differentiation' is the least reliable and lymphovascular invasion (LVI) is the most reliable predictor of nearby lymphnode micro-metastases.
This polyp on a short stalk was removed from the colon
WHAT IS THE LIKELY HISTOLOGY?
a) TA
At the edge, the crypts are clearly sli-like but how about the centre?
b) TVA
Doesn't look like gyrate crypts!
c) VA
Doesn't look like a sea anenome!
d) TSA
TSA's do have crypts but this lesion doesn't in the centre!
e) Early cancer
Was my own firm diagnosis!
explanation
The head of the lesion is clearly of concern. There are 'horns' on it! Of course, the devil has horns but as it was arising from a stumpy stalk. I went ahead and removed it. Somewhat surprising our pathologists called the lesion TA+HGD!
Admittedly, there was disagreement between our histopathologists and 2 out of 5 believed that it was an invasive cancer. Endoscopically, the lesion is clearly malignant and at the very least it's an intramucosal cancer (IMca). Of course, our UK histopathologists are unable to make the diagnosis of Intramucosal cancer in the colorectum because this is not a diagnosis recognised by the 'Vienna classification'. Elsewhere in the GI tract, intramucosal cancer is a diagnosis which our pathologists are 'free' to make. It makes no sense whatsoever to me !
This was found on the anterior gastric wall in an elderly patient with iron deficiency anaemia
WHAT IS THE MOST LIKELY DIAGNOSIS?
■ Gastric ulcer scar
Quite possible but you would take samples surely?
■ Gastric erosion
But there is a nodule in the centre?
■ Benign gastric ulcer
But there is no 'ulcer' in the centre?!
■ Early gastric cancer
Surely, the most likely diagnosis?
explanation
Gastric folds are being pulled into this 'lesion'. Of course most EGC's are depressed or flat elevated with a central depression. This is not easy to classify into the Paris classification system. I guess that you can argue that its' an elevated lesion with a central depression? Anyway, at the centre of the lesion there is a nodule! Not an ulcer! It's most likely an EGC. In fact, the lesion was resected endoscopically and proved to be poorly differentiated invasive cancer with a positive deep margin ! As the patient was 84 yrs old, no surgery was offered. That was 6 years ago and the patient remains well with only an unremarkable scar in the stomach to remind him ! Wonderful !
This patient was referred for a flexible sigmoidoscopy because of PR bleeding. However, the only abnormality found was a sore anal canal. Samples are taken of course.
HOW WOULD YOU NOW ADVICE THE PATIENT?
■ We'll see you in clinic once histology is to hand
But there is a degree of urgency here!
■ Symptoms may improve with 'anusol'
No they will not!
■ We will try topical mononitrate first
You are barking up the wrong tree!
■ We will organise a scan next
Yes, a rectal MRI revealed something important!
■ Inject 80mg of triamcolonone
You are missing the point!
explanation
Actually, this isn't a case of haemorrhoids or an anal fissure. Histology reported; " Within hyperkeratotic epidermis there are scattered individual highly atypical infiltrating malignant cells with frequent apoptosis and moderate clear cytoplasm. There is no ulceration or significant inflammation."
Actually, this is a case of Perianal Paget's disease, - a VERY rare condition!!! You'll remember that a 'puckering' of the skin around the breast areola is associated with underlying breast cancer. This was first reported by Sir James Paget in 1874. However, a few years later, the same phenomenon was described elsewhere, so called "extramammary Paget's disease". In descending order of frequency, this has been described at; the skin of the vulva, perineal skin, perianal skin and the skin of the scrotum. Paget's disease, is usually NOT a primary cancer of the apocrine glands of the skin. It's almost always secondary to a nearby cancer of the rectum, anus or prostate. In this particular case, further imaging revealed a nearby prostate cancer! ! This patient attended for dilatation of his biopsy confirmed peptic oesophageal stricture. Clip has been speeded up somewhat. WHAT WOULD YOU ORGANISE NEXT?
■ A clinic appointment
To ask pt about the swallowing? Hmm, somethings missing!
■ Another dilatation in 2 weeks time
Stricture not very tight and the dilatation should last longer
■ an early follow up OGD for biopsies
The mid-oesophageal stricture is peptic but what of the mucosa below?
explanation
The background to this case is a recent audit which we did in Leeds on missed upper GI neoplasia. We have had several instances of oesophageal cancers being missed when the endoscopist focused too much on the 'task in hand'. For example, we have had several SCC's missed when the endoscopist was carrying out a Barrett's surveillance endoscopy. This is another example of a missed (intramucosal) adenocarcinoma, glimpsed about 10 seconds into the clip in the 3 O'clock position. With mid-oesophageal peptic strictures there is often a stretch of Barrett's below which of course must be assessed and sampled at the earliest convenience. Of course, one can argue that when the job is to do something therapeutic, such as placing a PEG or removing a large polyp, it is 'permissible' to miss a cancer elsewhere. After all, the objective is not to undertake a careful diagnostic examination but to 'do a job'! I'm a strong believer that 'diagnostic' examinations and 'therapeutic' examinations must be clearly separated when you are looking at 'missed lesions. There is a distinct 'therapeutic window' during all endoscopic procedures done without a general anaesthetic. In the upper GI tract, it's up to 20-30 minutes and in the colon I think that it's up to 45-60 minutes. Your patient will not thank you for wasting the valuable minutes of your therapeutic window on carrying out a full diagnostic examination. Of course, after your therapeutic procedure you should consider if an early diagnostic gastroscopy or colonoscopy is needed. But carrying out a full assessment of the squamous portion of the oesophagus at the time of a Barrett's surveillance examination doesn't add much to the procedure. Similarly, when a mid-oesophageal peptic stricture is found, one should realise that the reason that the peptic stricture is in the middle of the oesophagus and not the gastro-oesophageal junction, is probably that there is a a Barrett's segment below the stricture. It doesn't add much time to the procedure and that IMca could have been spotted earlier! Previous biopsies have confirmed that this rectal polyp harbours TVA+HGD. It's removed by piecemeal EMR and at the end of the 2min video clip you see the end result. WHAT WOULD YOU DO NEXT?
■ Place clips
Always clips !
■ Wait for histology
And of course, you have requested an 'urgent report'!
■ Request EUS
Life is too short for EUS!
■ Organise an MRI
For local staging and confirmed T2,N1 cancer
■ Request staging CT
Looking for mets in the Chest+Abdomen+Pelvis - proved negative!
explanation
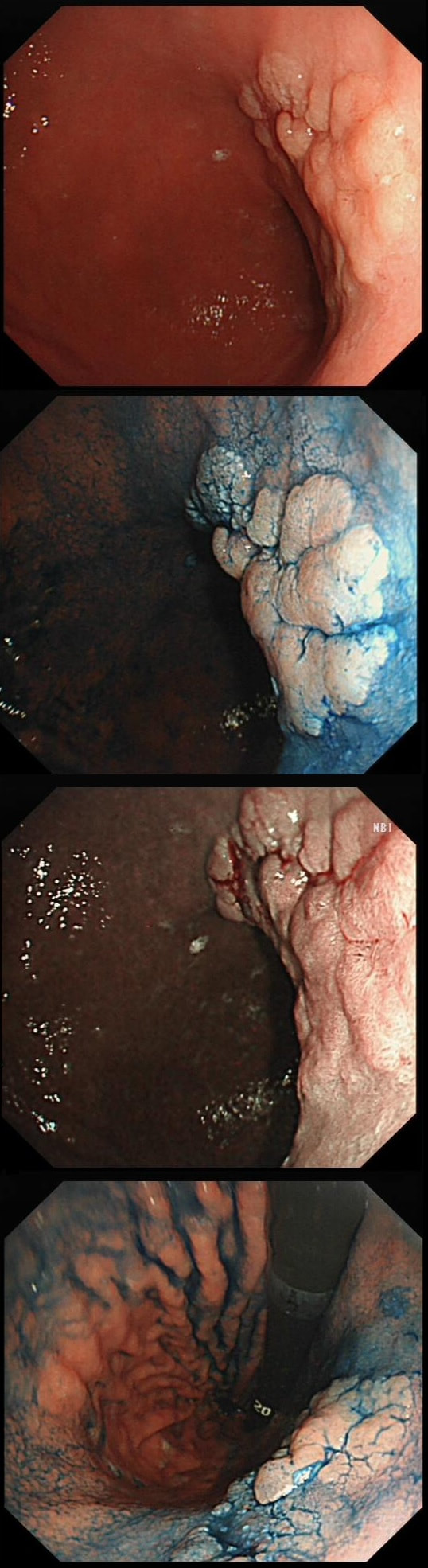
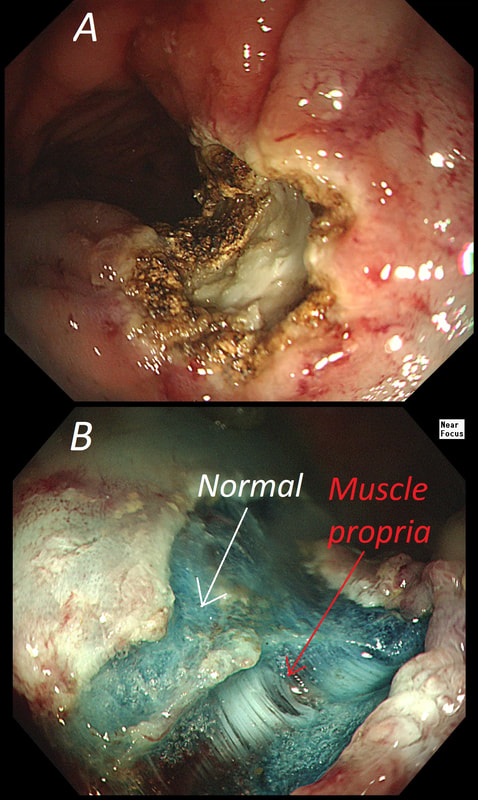
The learning point of this video clip is the EMR defect; it's white (image A) !!! Perhaps the picture below explains it better. Normally my EMR defects are blue (because I mix indigo carmine dye into the submucosal mix). In 'image B' below, the white arrow in the second image shows you what a 'healthy EMR defect should look like. IF you cut too deep, you can see the white, linear fibres of the muscle propria layer (red arrow). Of course this is a warning sign that you MUST carefully close the defect with lots of clips. Actually, next to the tip of the red arrow you can see a black 'micro-perforation' where the full thickness of the muscle layer has been breeched. Naturally, this is were your first clips goes!
Anyway, the mucosal defect in the video clip is just - white, without any linear muscle fibres. This is fibrosis! I have seen fibrosis like this below large sigmoid polyps which have been yanked about with the forceful sigmoid peristalsis. However, the more usual reason for this appearance is that you are looking at the fibrous tissue below a cancer, called 'desmoplasia'. Consequently, if I see a fibrous tissue in the base of the lesion I would do the following: Place clips (because I always do) Fast-track the histology Organise an MRI Request staging CT (chest+abdomen+pelvis) The ultimate diagnosis? Histology confirmed that the polyp was malignant and the imaging (of course requested at the time of the resection), confirmed a T2, N1 carcinoma. A week later, we had a full diagnosis. Unfortunately, the patient turns out not to be a surgical candidate. There is rarely unbridled joy after the endoscopic removal of a CRC ... This sizeable polyp was discovered at the ileo-caecal valve and was referred for resection. Samples have not been taken to avoid tethering down making the endoscopic resection uneccessarily difficult. HOW WOULD YOU APPROACH THE LESION?
■ Back off and take some samples
Yes, and perhaps request a CT!
■ Attempt an EMR
You first need to decide what this is!
■ Consider removal by ESD
Can't be adenomatous, it's growing out of the TI!
■ Underwater EMR
Would also be ill advised
■ Refer for surgery
If this is what you think it is, then Yes!
explanation
In most cases, I would say 'if it lifts it will shift'. However, in this case I wouldn't bother with a test-lift. The reason is that the thing is growing out of the terminal ileum. You don't get adenomatous polyps growing out of the TI! This must be something else! In fact the true lesion may be larger than the red nipple-like polyp. Even though there are no large 'tell-tale' vessels running up it's side, the only thing of this size, growing out of the TI, is a NET! A lymphoma was my second guess. TI NET's are often bad news and should be considered for surgical resection. There is another odd thing about NET's growing in the terminal ileum. The WHO grading system doesn't seem to relate to the aggressiveness of the lesions behaviour ! This was only WHO grade I (proliferation index was only 1.8%) but on a full analysis after the right hemi-colectomy, the NET was found to be a invading into the muscle propria layer and with metastatic deposits in 2 out of 12 resected lymph nodes (T2,N1) as well as ulcerated deposits of NET in the nearby pericolic fat on the serosal surface. The moral of the story? In the terminal ileum, 'well differentiated NET' doesn't mean that it's well behaved! This lesion was found in the sigmoid. You have magnification, NBI, dye spray and lift to help you decide. WHAT IS THE STAGE OF THIS CANCER?
■ Intramucosal
There is no such thing!
■ sm1 invasion
You can see a disorganised crypt pattern?
■ sm2 invasion
That was my guess!
■ sm3 or 'massive' invasion
I think that the lift is better than that!
explanation
I think that it's difficult to tell the difference between no crypt pattern at all and a severely disrupted crypt pattern. Of course, when the crypt pattern is 'severely disorganised' but some of it is still visible, the lesion will be sm1 or sm2. In contrast, if there is no crypt pattern at all, the lesion is sm3 or beyond (the Japanese call this 'massive invasion'). Instead, I rather rely on the degree of lifting. In this case, the endoscopist decided that the lifting was insufficient for a resection and backed off, referring the patient to our MDT. Biopsies confirmed that the lesion was likely to be malignant and the patient ended up with sigmoid resection. I think that there is some sort of crypt pattern in the centre of the lesion. Furthermore, looking at the slight degree of lifting, my guess would be that the lesion is sm1 or sm2 and therefore potentially endoscopically resectable. Actually the cancer turned out to be sm2 (T1,N0). |
Categories
All
|