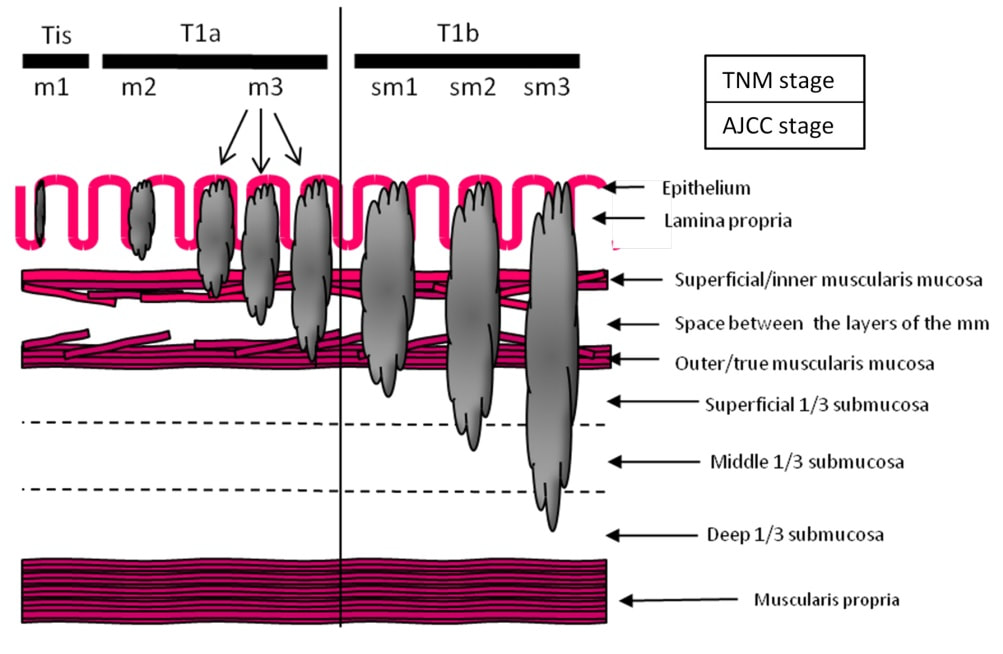
|
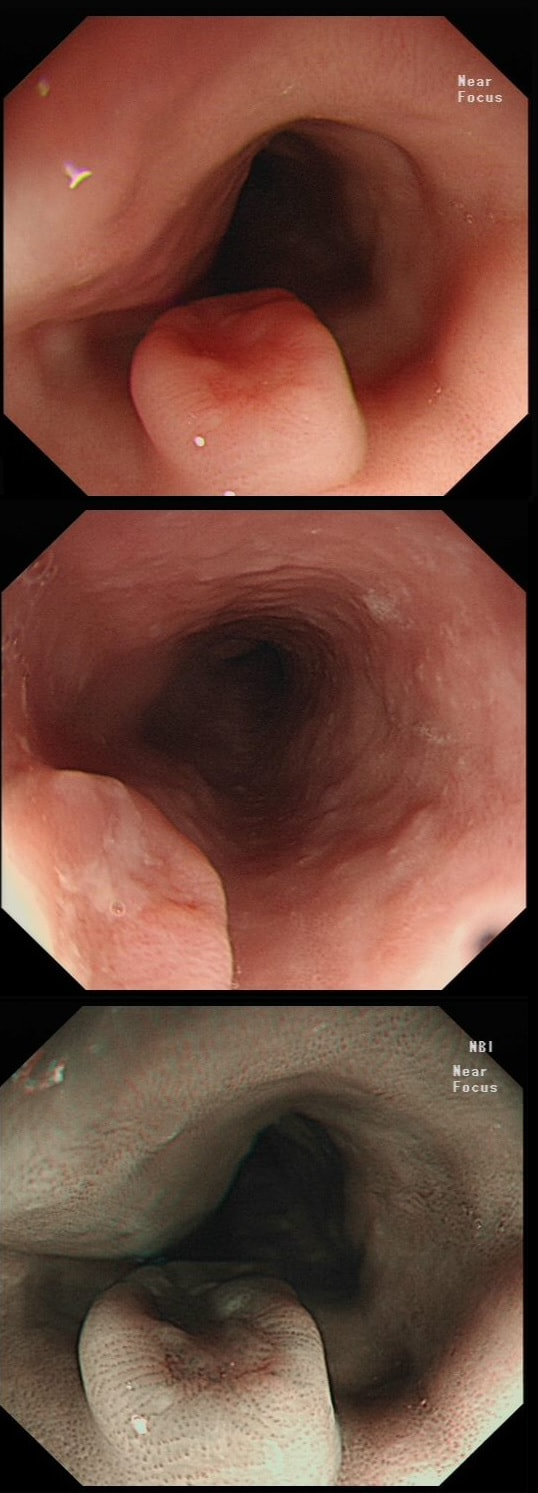
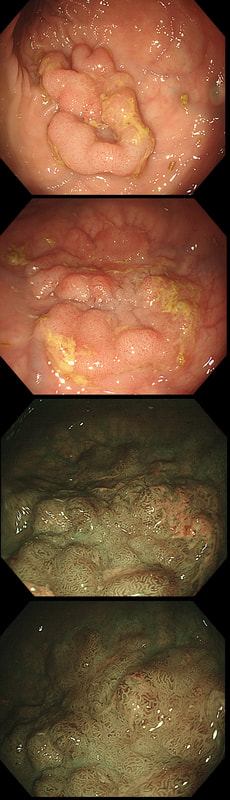
This is the stomach of a middle aged lady undergoing gastroscopy because of anaemia.
WHAT IS THE DIAGNOSIS?
a) HP associated gastritis
This is beyond gastritis! The fundal mucosa is pale and atrophic rather than red and inflammed!
b) Atrophic gastritis
The fundal mucosa does appear atrophic apart from a few scattered red patches which is all that remains of the normal gastric lining. But how about the antrum?
c) Autoimmune gastritis
Correct!
D) Coeliac disease
That duodenal mucosa is unremarkable. The 'polyp' in the 3 o'clock position is obviously the duodenal papilla.
Explanation
Those red spots in the gastric fundus is what remains of the more normal gastric mucosa whilst most of the surrounding mucosa is atrophic. In contrast, the antrum is unremarkable, as is the duodenum. This is an example of a 'body predominant' gastritis and your endoscopic diagnosis should be an autoimmune gastritis! The antral G-cells, found deep within the antral pyloric glands are pumping out huge amounts of gastric which is leading to ECL cell hyperplasia and multiple small NET's. In fact, most patients with type I gastric carcinoids have an autoimmune gastritis! The anaemia was due to vitamin B12 deficiency.
This 'lesion' was barely visible within in a Barrett's segment on white light. However, after acetic acid and with NBI it's more obvious.
WHAT IS THE LIKELY HISTOLOGY?
a) Barrett's LGD
Usually invisible or just a red, flat patch
b) HGD
Subtle change in crypt pattern but with little nodularity?
c) IMca
This was my own guess but it was wrong...
d) Invasive cancer
Yes, superficial invasion (100 microns) and poor differentiation!
explanation
I removed this lesion without worrying too much about the subtle 'depressed' growth pattern and the small, round crypts in the centre of the lesion. However, I was surprised to see the pathology report of a superficially invading adenocarcinoma, with poor differentiation to boot!!! This finding makes the advice on 'further treatment' more complex. As you know, in both the upper and lower GI tract, the finding of 'lymphovascular invasion' (LV) is probably the most 'ominous sign' that a patient needs surgery (or chemo-radiotherapy in case of the oesophagus). Poor differentiation is 'bad', but less bad than LVI.
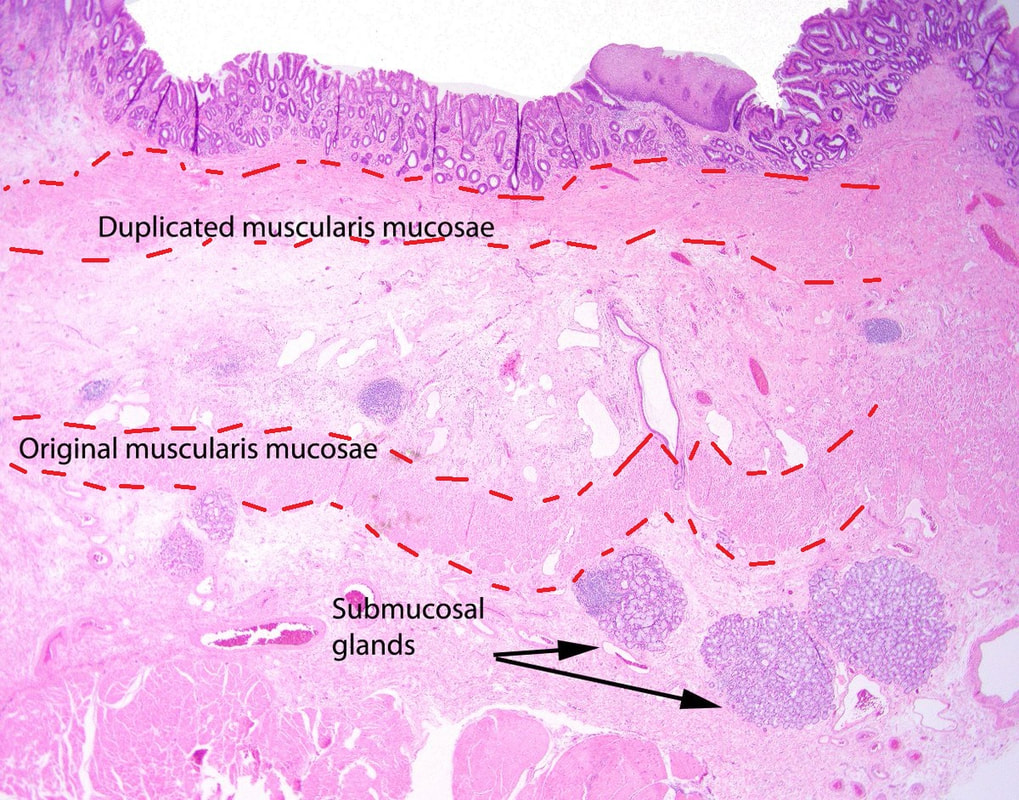
Depth of invasion is also important and in Barrett's you are 'allowed' invasion to about 500 microns below the muscularis mucosa. The corresponding 'safe margin' in SCC's is only 200microns. In this case the depth of invasion was only about 100 microns, leaving 'poor differentiation' as the only 'bad sign'. The patient wasn't a surgical candidate and refused CRT. This was 3 years ago and so far all is well! By the way, the histopathologists do have a more difficult job than you perhaps imagine, measuring the depth of invasion in Barrett's cancer. This is because they often see several bands of muscularis mucosa, so called 'duplication of the muscularis mucosa'. Elsewhere in the GI tract, the muscularis mucosa is a single band of smooth muscle. They measure the depth of invasion from the top of the muscularis mucosa down the the deepest point of invasion. However, if there are several bands of muscularis mucosa, which one do you measure from?!? Below is an example to illustrate the dilemma.
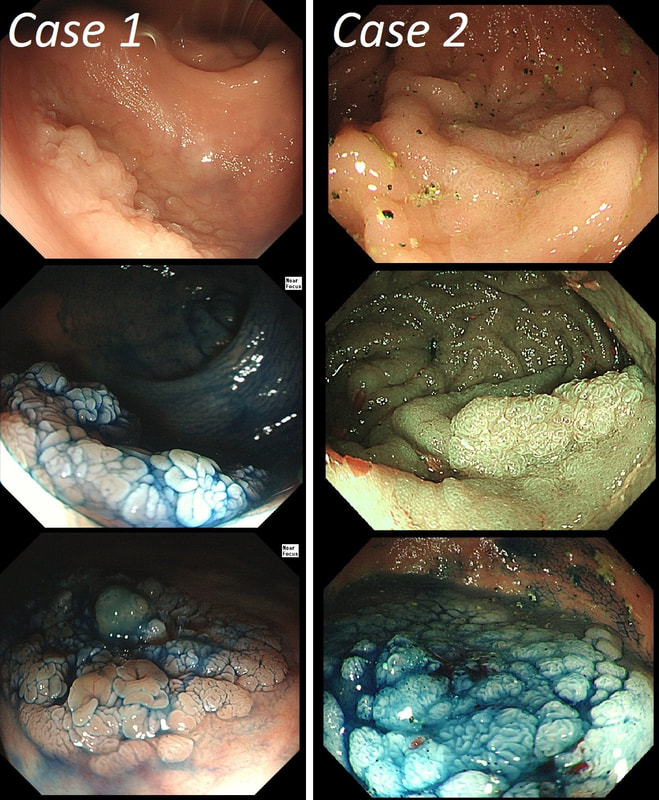
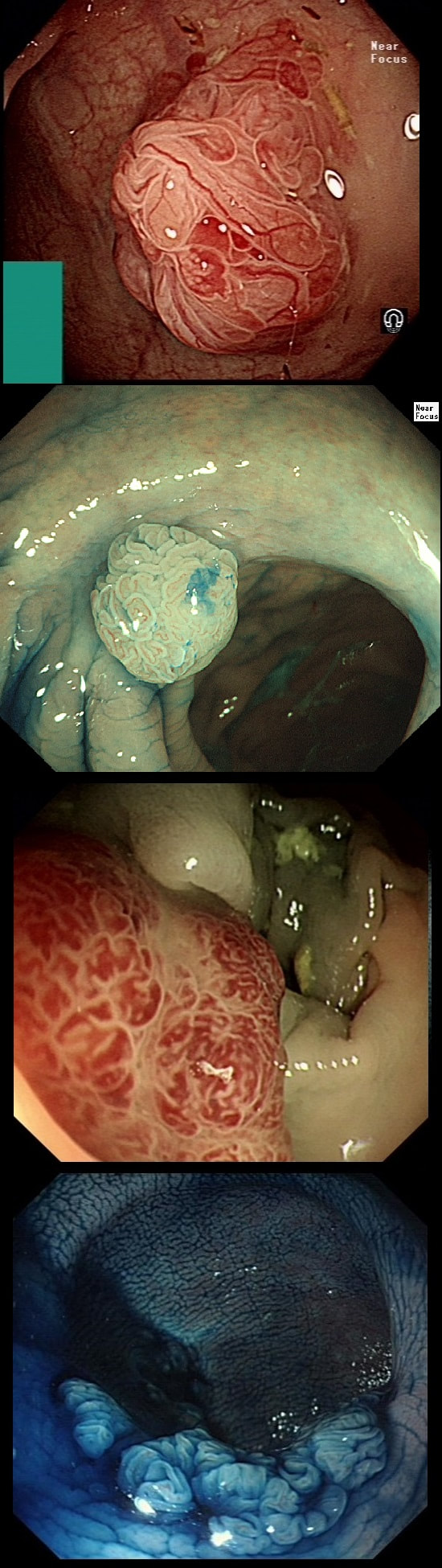
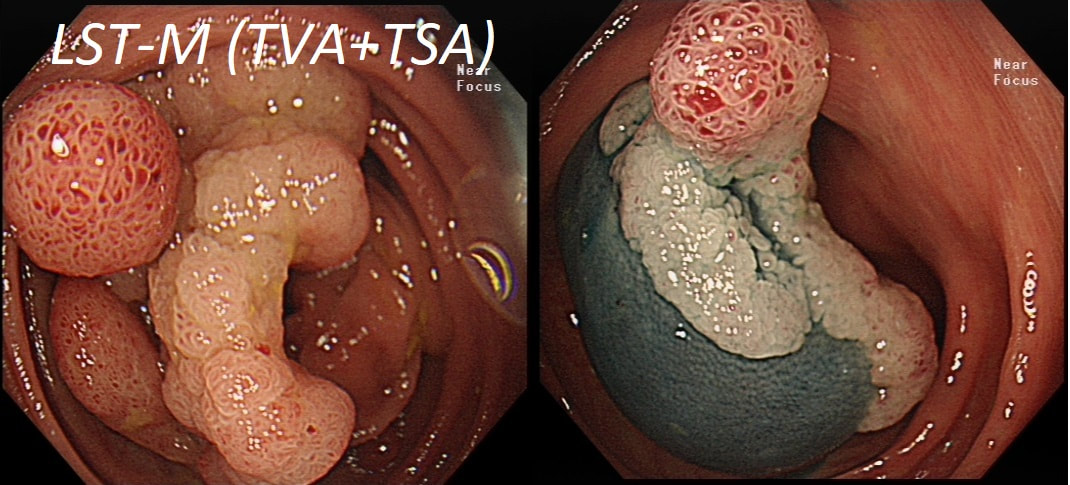
You may be surprised to hear that both these polyps (found in the caecum and rectum respectively) actually have the same histology!
WHAT IS THE LIKELY HISTOLOGY OF THESE TWO LESIONS?
a) SSL's
But it has gyrate crypts and perhaps villous in places??!
b) TSA's
Well done! TSA's are great cameleons !
c) TA's
Slit like or small round crypts? No !
d) TVA's
Reasonable guess as the growth morphology is that of a LST-G which are usually TVA's!
e) VA's
Right-hand image does look a little villous, perhaps
explanation
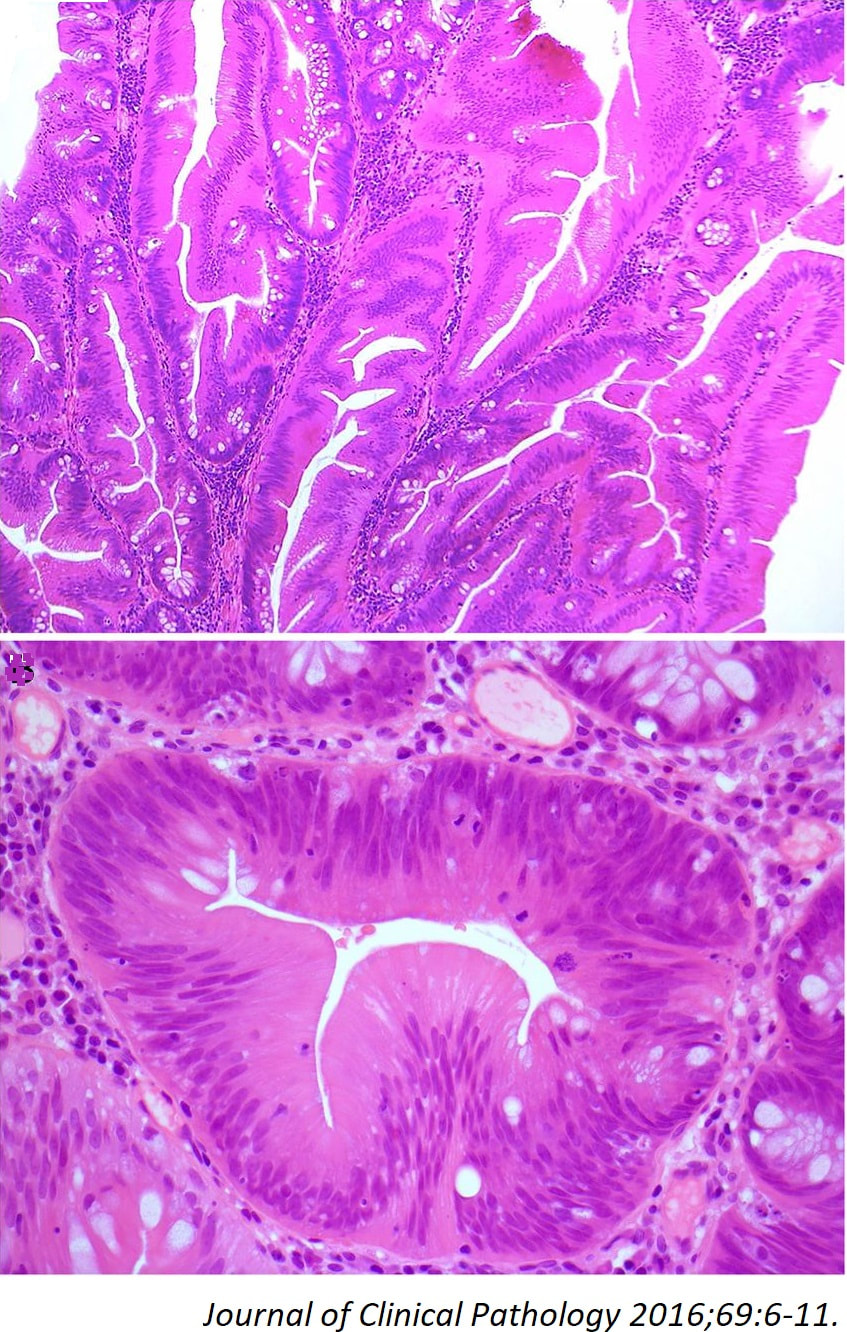
The surface appears villous in places and gyrate in other areas. The truth is that it is a bit of both - a 'Traditional Serrated Adenoma' (TSA), first described in 1990 by Longacre and Fenoglio-Preiser (Am J Surg Pathol 1990;14:524–37). TSA's are usually less recognisable and usually somewhat 'cerebriform' (like an exaggerated TVA).
TSAs are the least common of the three serrated colonic polyps ('hyperplastic polyps', 'sessile serrated lesions' and TSA’s) and account for only about 5% of serrated polyps and less than 1-2% of all colonic polyps and are usually found in the distal colon. Pathologists rely on three typical findings when diagnosing TSA’s: deeply eosinophilic cells, flat top luminal serrations and numerous ectopic crypt foci (all demonstrated in the histology slide below from the Journal of Clinical Pathology 2016;69:6-11). Genetically, TSA's are more like adenomatous polyps harbouring low-level microsatellite instability (MSI-L) or microsatellite stable (MSS) serrated colorectal adenocarcinomas. They usually contain KRAS mutations rather than usual BRAF mutations which you find in the 'Serrated Pathway' to CRC associated with a high-level of microsatellite instability (MSI-H). Nevertheless, it’s thought that these precursor lesions give rise to serrated colorectal adenocarcinomas as part of the serrated (accelerated) pathway to cancer. This lesion in the low rectum has been referred for endoscopic resection WHAT IS YOUR DIAGNOSIS?
a) Mucosal prolapse syndrome
Well done!
b) Sessile Serrated lesion
Looks just like it but it isn't!
c) Traditional Serrated Adenoma
That's a No!
d) Adenomatous polyp
Patient complained of constipation...
e) Malignant polyp
But it's not 'chunky', red and it has crypts ?!?
explanation
There is no distinct edge to the 'polyp' and the crypts look normal, although a little splayed out and larger than normal, reminiscent of a serrated polyp. In real life, I had the advantage of knowing that the patient had been sent for colonoscopy because of constipation. I low-risk indication of course. There is no distinct edge to the 'polyp' and the crypts look normal, just a little splayed out and larger than normal. This 'polyp' is actually part of the "Mucosal prolapse syndrome", first described in 1983 by Du Boulay et al [J Clin Pathol. 1983;36:1264–8]. Obviously, this syndrome includes solitary rectal ulcers, inflammatory cloacogenic polyps but also rectal 'prolapse polyps' such as this. In addition, the 'Mucosal Prolapse Syndrome' includes those prolapsing mucosal folds in the sigmoid and perhaps most surprisingly - GAVE ! In a case like the above, a couple of superficial biopsies will usually reveal the true nature of the lesion as it's easy for pathologists to spot the fibromuscular hyperplasia with overlying epithelial crypt distortion rather than dysplasia.
Patient was complaining of indigestion and reflux symptoms.
WHAT IS YOUR DIAGNOSIS?
a) Normal oesophagus
Not quite normal is it?
b) Reflux oesophagitis
This doesn't look like reflux!
c) Adenocarcinoma
Actually the correct answer!
d) SCC
Red nodules, even if surrounding by squamous mucosa are adenocarcinomas (usually)
explanation
You may be surprised to hear that the small nodule at 3 O'clock turned out to be an IMca! It was removed endoscopically. Of course, it's the question then arises; "Should we offer RFA". The BSG recommends this for patients with Barrett's harbouring flat dysplasia. However, in this case there is only a tiny, tongue of Barrett's in the 6 O'clock position! Actually, we just gave this a quick blast of APC (at a fraction of the cost of RFA) and it was gone. However, the patient remains on annual surveillance!
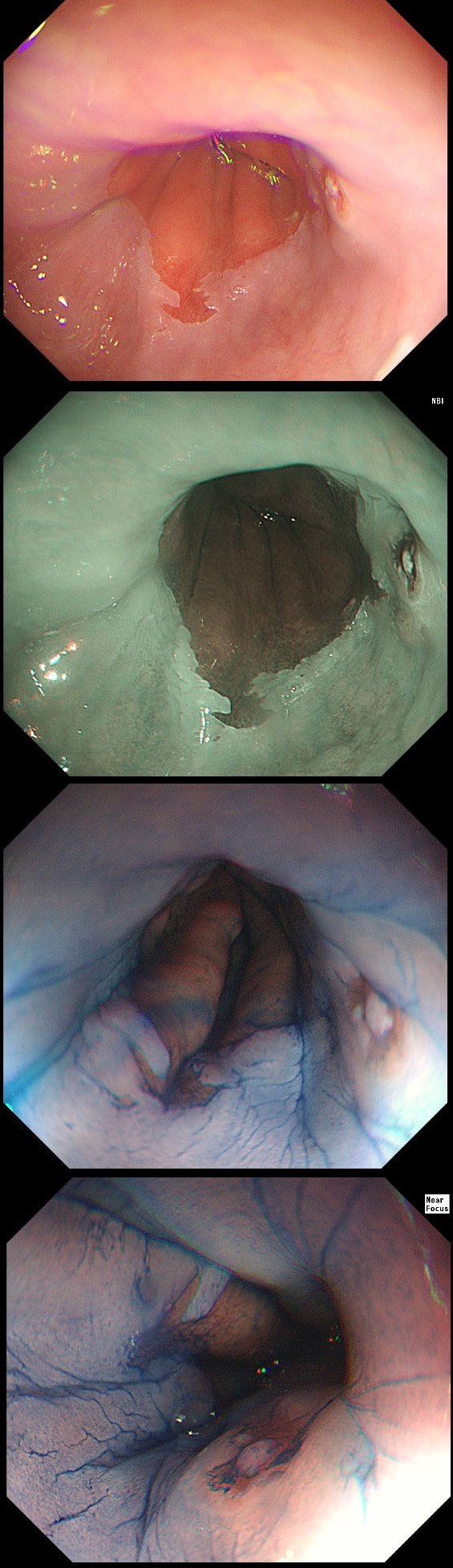
An odd lesion in the middle of the oesophagus of a 55 year old woman. WHICH OF THESE POSSIBILITIES IS THE MOST LIKELY?
a) Papilloma
Papillomas look more warty and I've never seen them ulcerate
b) Leiomyoma
Statistically the most likely benign lesion probably!
c) GIST
Are very rare in the oesophagus!!!
d) NET
Are also very rare in the oesophagus!
e) SCC
Not likely as the IPCL's are regular and the lesion isn't brownish!
explanation
GIST's are extremely rare in the oesophagus whilst common in the stomach. In contrast, leiomyomas are common in the oesophagus and rare in the stomach. However, this looks odd and the previous biopsies had not been able to get to the bottom of the diagnosis. Are we missing a SCC? However, it looks like something from deeper layers were breaking through the mucosal surface. In contrast an SCC should start growing at the surface and in time invade deeper. The 'intrapapillary capillary loops (IPCL's) dilate up, become irregular and eventually completely disorganised in SCC's. At the same time the oesophageal wall takes on a brownish tint on NBI.
I decided to remove it and it turned out to be a leiomyoma. I presume that it had got traumatised or perhaps by pressure necrosis ended up with a funny surface. Don't think that I have seen an oesophageal NET as yet. Presumably they are very rare in this location. By the way, I've uploaded an example of a T2 mid-oesophageal SCC in the clip below. The lesion looks smallish but is already beyond endoscopic cure and Lugol's shows up a large area of background dysplasia which looks orange rather than brown after Lugol's dye spray.
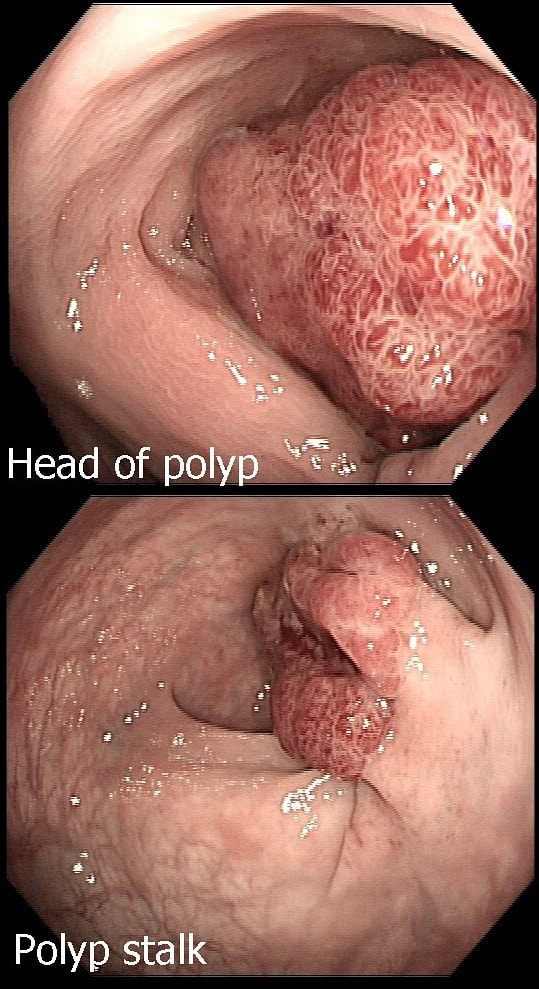
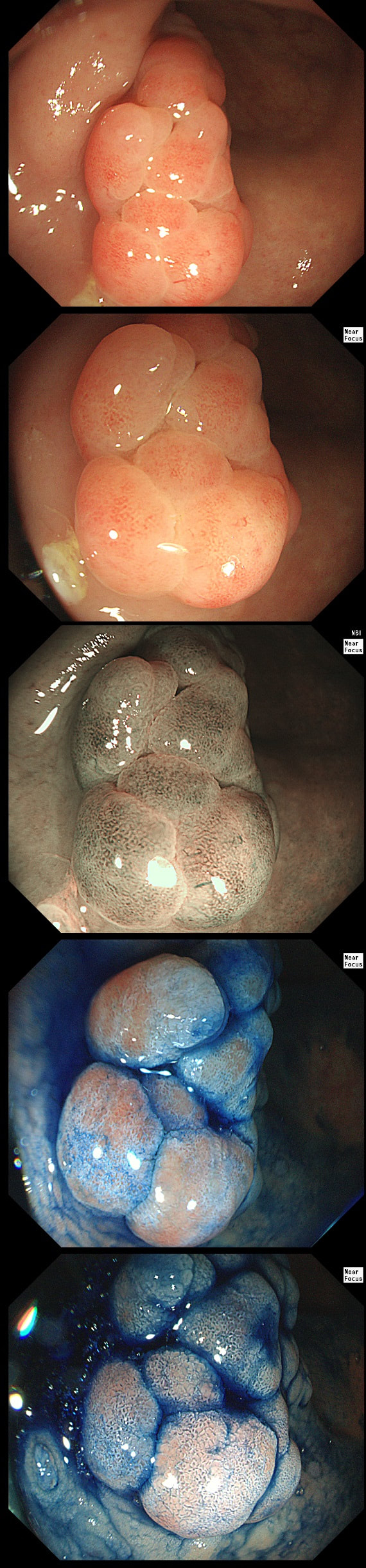
This is an odd looking sigmoid polyp which is seen arising from a broad stalk.
WHAT IS YOUR ENDOSCOPIC DIAGNOSIS?
■ Tubular adenoma (TA)
But the crypts don't look slit-like do they?
■ Tubulovillous adenoma (TVA)
Was my first thought but it doesn't look quite right...
■ Villous adenoma (VA)
If your bx forceps disappears into the polyp it's a VA
■ Traditional Serrated adenoma (TSA)
You'd be excused if you got this wrong - these are rare beasts!
explanation
My initial endoscopic diagnosis was that of a TVA. However, it looked a little "exaggerated". Actually it turned out to be a "traditional serrated adenoma" (TSA).
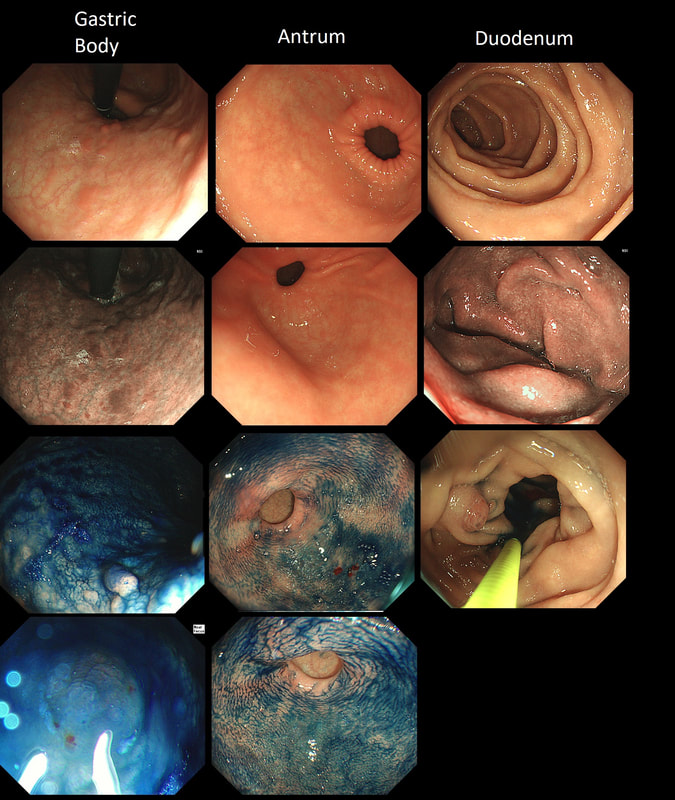
TSA's are rare and mysterious polyps. They only accounting for about 1% of colorectal polyps and are most common in the sigmoid and rectum. Endoscopically they can appear as “exaggerated” tubulo-villous adenomas or as villous adenomas. In the image below, I've put four examples of TSA's together to illustrate how differently these can appear ! It has been proposed that because one-third of "serrated cancers" are found in the distal colon and perhaps these cancers arise from TSA’s! However, genetically, TSA's are more like adenomatous polyps harbouring low-level microsatellite instability (MSI-L). They usually contain KRAS mutations rather than the BRAF mutations which you find in the 'Sessile Serrated Lesions'. For this reason, it's perhaps unlikely that they contribute to the 'Serrated Pathway' to cancer. By the way, a recent paper has highlighted that patients with TSA's are also at greater risk of 'high risk polyps', elsewhere in the colon.
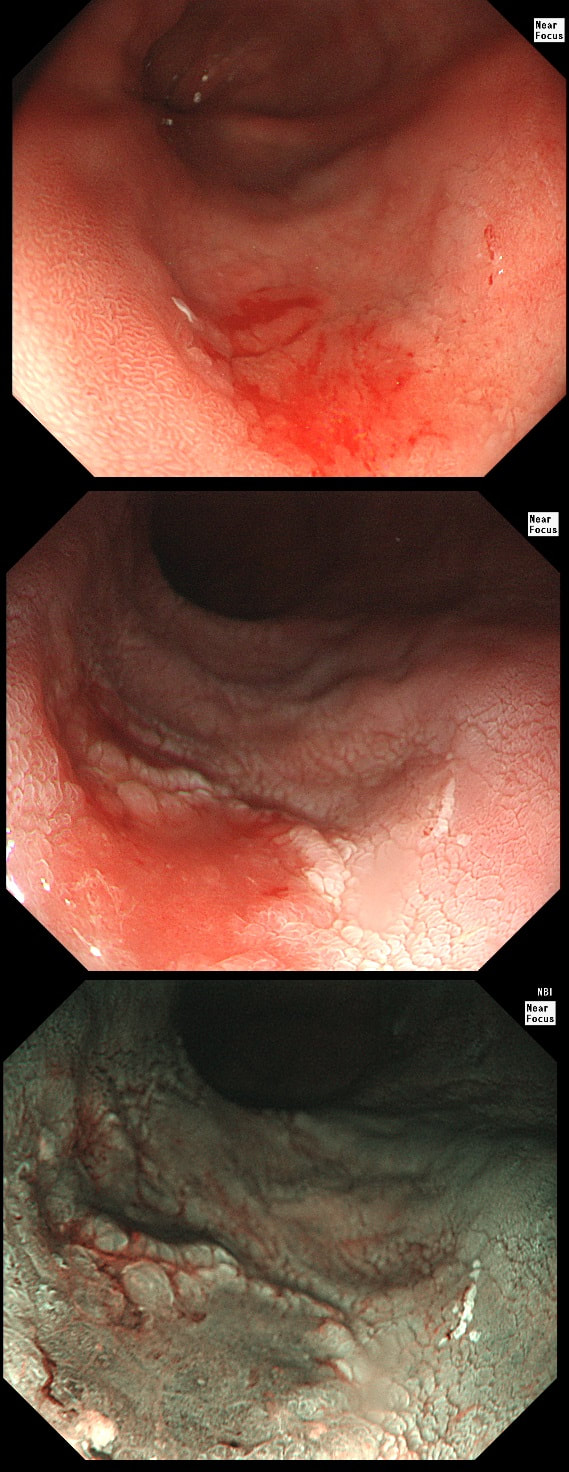
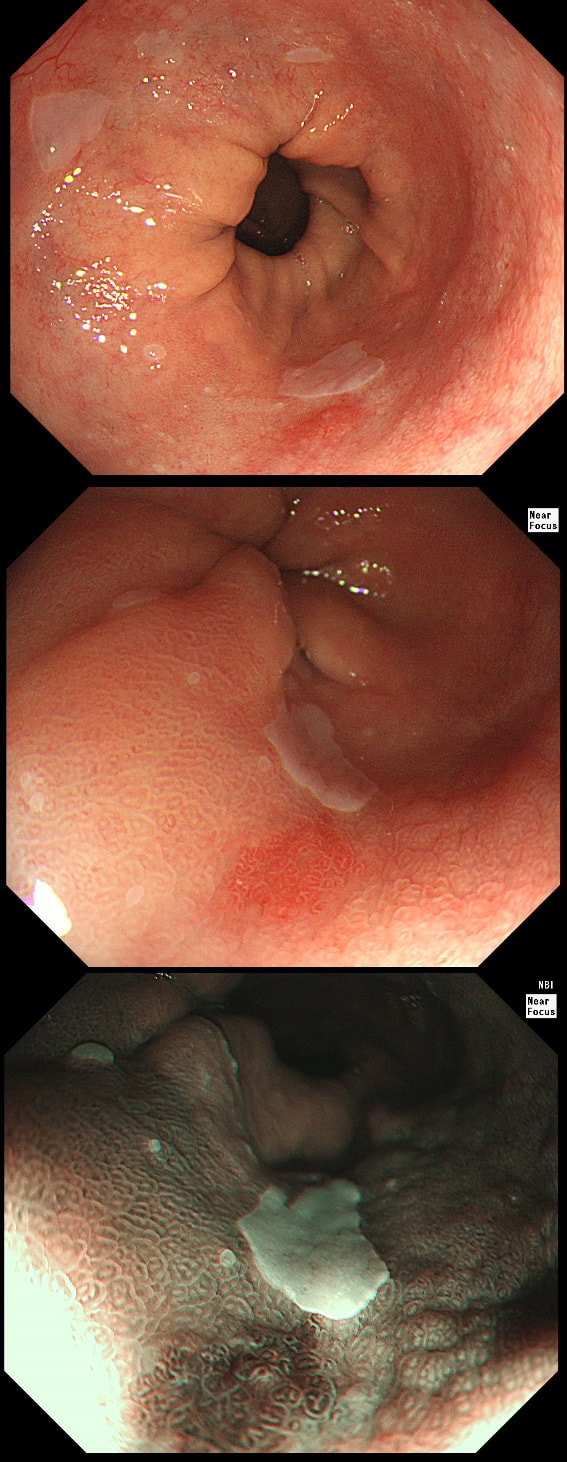
During a Barrett's surveillance OGD, I notice this small red spot at 6 O'clock.
WHAT IS THE LIKELY HISTOLOGY?
■ Reflux ulcer
I don't think that you get this within Barrett's without dysplasia!
■ LGD
Clear crypt pattern, not that different from surrounding mucosa? Good guess!
■ HGD
Quite possible and would have been my guess IF there was some nodularity associated
■ Early cancer
Impossible as lesion has an organised crypt pattern!
explanation
Previous surveillance samples, 3 years earlier had not revealed any dysplasia. However, that red spot shouldn't be there! A reflux ulcer is unlikely as the Barrett's is there because it protects the food pipe from acid. Of course it does this by developing crypts with Goblet cells which secretes protective mucus (of course the pathologists call this process 'metaplasia'). Early texts usually stated that ulceration is common within Barrett's. In my experience, ulceration within Barrett's only happens when dysplasia develops. With the arrival of dysplasia, you begin to see a disappearance of the Goblet cells and of course when the Goblet cells start to dwindle, so does the protective mucus.
In the bottom zoom image you can see that there is a distinct surface crypt pattern at the site of the red spot. Therefore the cells can't be too disorganised, making cancer unlikely. Actually this turned out to be a spot of LGD. I would have guessed HGD/IMca (pathologists struggle to tell the difference) if there was a visible nodule at the site of the red spot. Isn't it interesting that even at the very earliest stage of dysplasia, the unstable cells signal the need for more oxygen to nearby vessels?
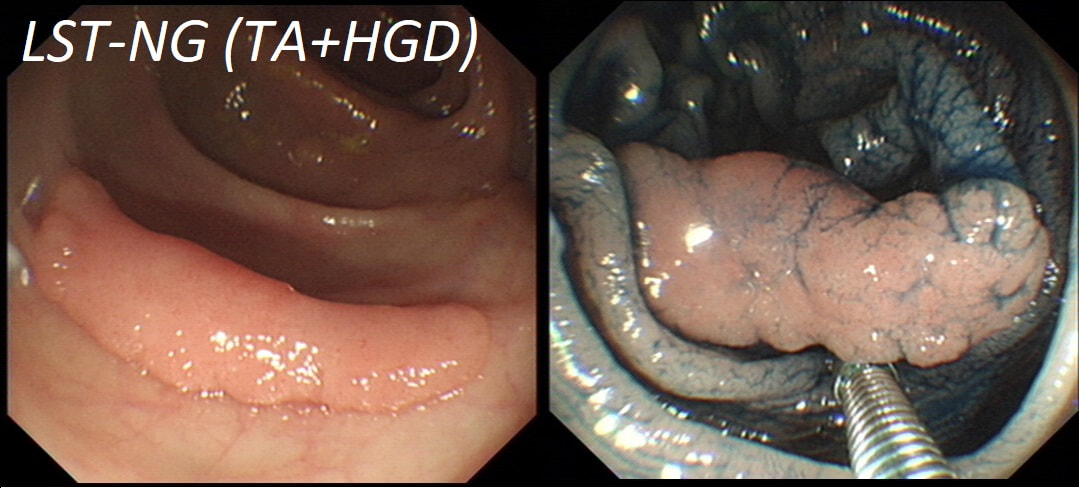
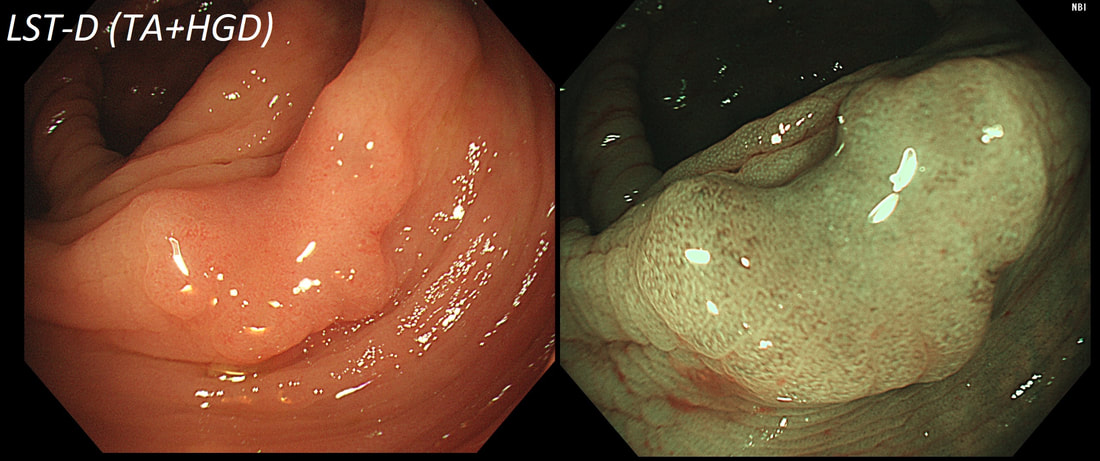
This lesion was found in the ascending colon.
HOW WOULD YOU DESCRIBE IT?
■ Flat elevated lesion (IIa)
Only lesions 10mm or smaller are referred to as 'IIa lesions'
■ Flat elevated lesion with a central depression (IIa+IIc)
But that depression dissappear when lumen is inflated!
■ Laterally spreading tumour of the non-granular type (LST-NG)
But the surface is 'cobbled' not smooth!
■ Laterally spreading tumour with a central depression (LST-D)
LST-D's are always TA's (and therefore have a smooth surface)
explanation
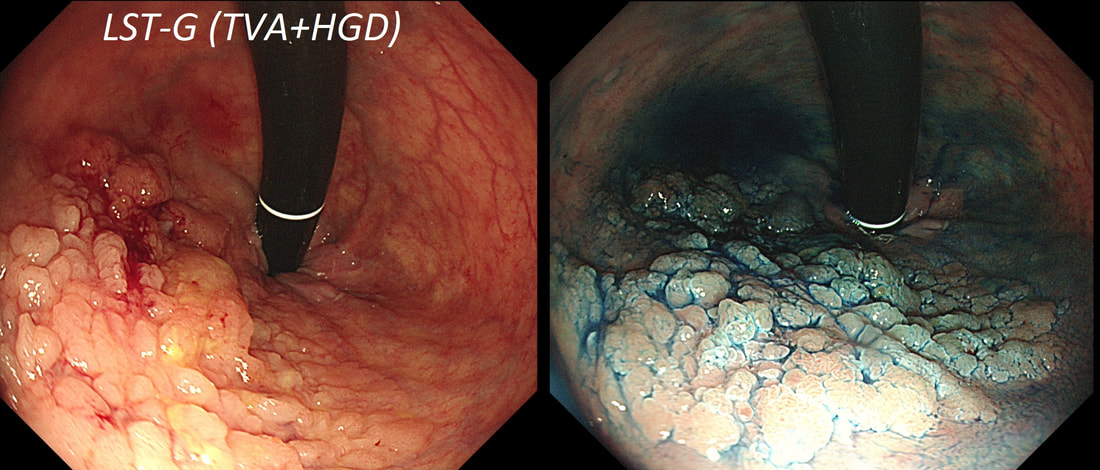
Lets just state something from the start! A flat elevated polyp is of course a IIa type of lesion BUT when larger than 10mm in diameter is no longer referred to as a IIa lesion. Now, it's a 'Laterally Spreading Tumour'!
There are 4 types as follows:
In this case the crypts are gyrate, (type IV crypts), making it a TVA. Laterally Spreading Tumours with a gyrate crypt pattern are all TVA's and almost never harbouring more than LGD. OK but it looks depressed in the centre doesn't it? No, it isn't depressed in the centre! It's simply they way the large lesion has folded itself. This is something of a 'trick question' as I deliberately didn't inflate the colonic lumen fully in the first image. When the lumen is inflated, you can see the true shape of the lesion; a LST-G ! A meta-analysis in 2013 by Voorham et al. [Voorham QJM. AM J Gastro 2013;108(7):1042-] concluded that pedunculated polyps were more likely to harbour KRAS mutation and APC mutations than flat lesions, and that flat lesions were more likely to harbour BRAF mutations. Depressed lesions and LST-NG’s were particularly unlikely to contain KRAS. Below are some examples of LST's This sizeable polyp was discovered at the ileo-caecal valve and was referred for resection. Samples have not been taken to avoid tethering down making the endoscopic resection uneccessarily difficult. HOW WOULD YOU APPROACH THE LESION?
■ Back off and take some samples
Yes, and perhaps request a CT!
■ Attempt an EMR
You first need to decide what this is!
■ Consider removal by ESD
Can't be adenomatous, it's growing out of the TI!
■ Underwater EMR
Would also be ill advised
■ Refer for surgery
If this is what you think it is, then Yes!
explanation
In most cases, I would say 'if it lifts it will shift'. However, in this case I wouldn't bother with a test-lift. The reason is that the thing is growing out of the terminal ileum. You don't get adenomatous polyps growing out of the TI! This must be something else! In fact the true lesion may be larger than the red nipple-like polyp. Even though there are no large 'tell-tale' vessels running up it's side, the only thing of this size, growing out of the TI, is a NET! A lymphoma was my second guess. TI NET's are often bad news and should be considered for surgical resection. There is another odd thing about NET's growing in the terminal ileum. The WHO grading system doesn't seem to relate to the aggressiveness of the lesions behaviour ! This was only WHO grade I (proliferation index was only 1.8%) but on a full analysis after the right hemi-colectomy, the NET was found to be a invading into the muscle propria layer and with metastatic deposits in 2 out of 12 resected lymph nodes (T2,N1) as well as ulcerated deposits of NET in the nearby pericolic fat on the serosal surface. The moral of the story? In the terminal ileum, 'well differentiated NET' doesn't mean that it's well behaved!
This lesion was found in the rectum of a patient undergoing colonoscopy because of constipation
WHAT IS THE LIKELY DIAGNOSIS?
■ Polyp from mucosal prolapse
Clever!
■ Serrated polyp
Crypts look a little serrated but the rest doesn't
■ Adenomatous polyp
Polyp doesn't look 'neoplastic'
■ Malignant polyp
Now, this doesn't look malignant!
explanation
Well perhaps the crypts look a little like serrated crypt openings but somehow the rest of the polyp doesn't look like a typical serrated lesion. Where is the covering mucus?! Furthermore, the polyp definitely doesn't look adenomatous or malignant!
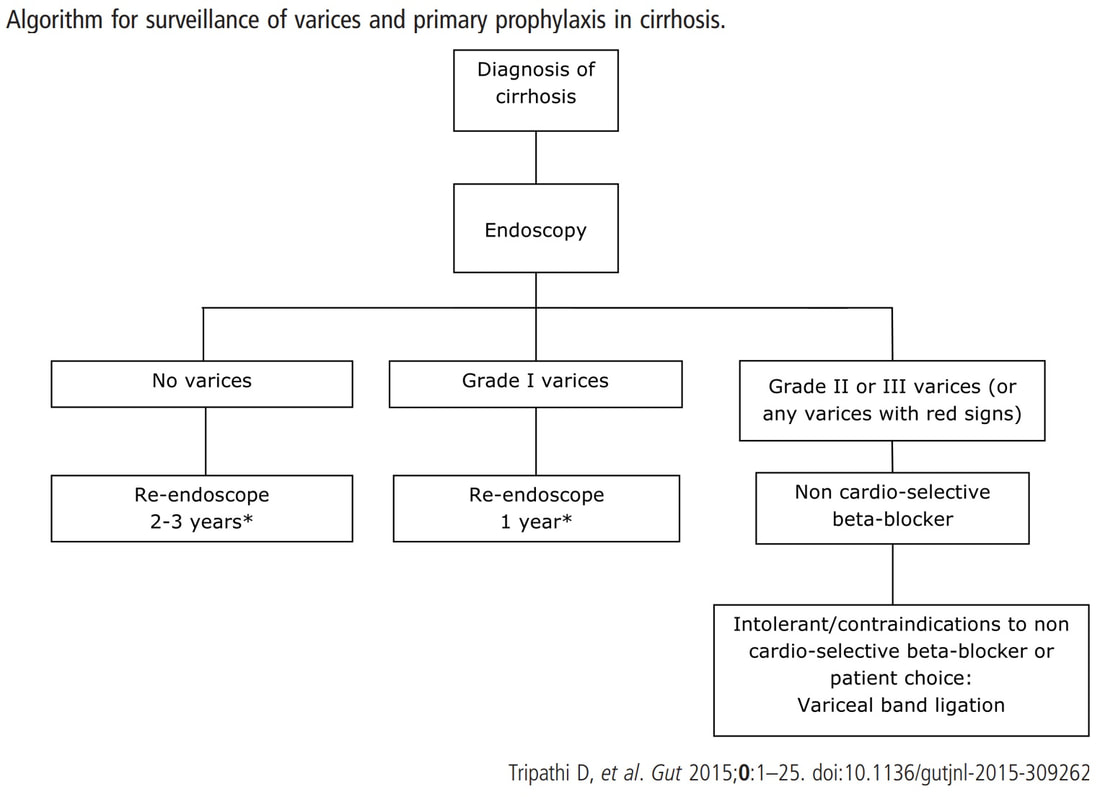
Actually this has arisen as part of the 'Mucosal prolapse syndrome' which is the umbrella term for entities such as; solitary rectal ulcer syndrome and inflammatory cloacogenic polyps. Patients are often constipated or have difficulty with defaecation with straining on the toilet or undergo the sigmoidoscopy because of tenesmus, altered bowel habits or incontinence. Surprisingly, some patients don't have any straining-related complaints! Most pathologists would recognise the typical mild fibrosis, thickening of the muscularis mucosae and crypt irregularity (dilated, diamond shape crypts). The surface epithelium show regenerative changes. This patient is on a surveillance programme due to alcoholic liver disease but has never had a bleed. He is maintained on a non-cardioselective β blocker. WHEN WOULD YOU RECOMMEND THE NEXT SURVEILLANCE EXAMINATION?
■ None, this patient should be offered banding
Correct, if you decide that there are 'red signs' or the varices are grade II or III
■ In 12 months time
Correct, if you decide that there are no 'red signs' and that the varices are grade I
■ In 2-3 years time
Would only be correct if there are no varices at all!
explanation
I must admit that I don't like the current surveillance guidelines for patients with portal hypertension. This is a good example why! Although the LFT's had remained stable and the patient had remained abstinent, you could argue that there are 'red signs'. Of course, the presence of 'red signs' predicts progression of varices [J Hepatol 2003;38:266–72] and because the patient is already on a β-blocker, band ligation should now be started. The truth is that 'red signs' are common and have a poor agreement value between endoscopists.
If you decide that there are no 'red signs', and that the varices are grade I only (which depends on the degree of inflation of the oesophagus), the recommendation is to offer surveillance in 1 year. Finally, if you decide that the varices are now grade II (or III), variceal band ligation would be the logical next step. Thus, you can make an subjective argument for any of the above three treatment options. Of course, what you decide will be judged in hindsight. If you decide that these are not red signs and the patient re-presents with a bleed in 6 months time, you could be open to criticism for missing signs of progressive liver disease. Surely, in the modern era of FibroScans, it's possible to predict progression of portal hypertension non-invasively!!! Five years ago, the BAVENO VI workshop only mentioned in passing, that surveillance endoscopies may be avoided in patients with elastography values <20 kPa and a platelet counts >150,000 as these patients are at low risk of progression. Similarly increasing size of the spleen is another warning sign and could be looked for when these patients attend for screening for HCC's. The American guidelines suggest that there is no need to offer patients with untreated viral cirrhosis a screening endoscopy to search for varices IF elastography is <20 kPa and the platelets are >150. They concede that annual elastography and platelet counts may be less predictive in other causes of cirrhosis. However, the American guidelines advice continued surveillance if varices have been found in the past, particularly if liver injury is ongoing. The next Baveno conference in October 2021 will hopefully recommend non-invasive monitoring rather than endoscopy. It would be cheaper, less arduous for patients and offer less subjective findings! The now rather dated BSG guidelines are summarised in the graph below. This lesion was found in the sigmoid. You have magnification, NBI, dye spray and lift to help you decide. WHAT IS THE STAGE OF THIS CANCER?
■ Intramucosal
There is no such thing!
■ sm1 invasion
You can see a disorganised crypt pattern?
■ sm2 invasion
That was my guess!
■ sm3 or 'massive' invasion
I think that the lift is better than that!
explanation
I think that it's difficult to tell the difference between no crypt pattern at all and a severely disrupted crypt pattern. Of course, when the crypt pattern is 'severely disorganised' but some of it is still visible, the lesion will be sm1 or sm2. In contrast, if there is no crypt pattern at all, the lesion is sm3 or beyond (the Japanese call this 'massive invasion'). Instead, I rather rely on the degree of lifting. In this case, the endoscopist decided that the lifting was insufficient for a resection and backed off, referring the patient to our MDT. Biopsies confirmed that the lesion was likely to be malignant and the patient ended up with sigmoid resection. I think that there is some sort of crypt pattern in the centre of the lesion. Furthermore, looking at the slight degree of lifting, my guess would be that the lesion is sm1 or sm2 and therefore potentially endoscopically resectable. Actually the cancer turned out to be sm2 (T1,N0). This polyp was found in the transverse colon of a 60 year old man. WHAT IS THE LIKELY HISTOLOGY OF THE LESION?
■ Serrated polyp
Crypt openings look a little serrated but nothing else does!
■ Adenomatous polyp
This is not an adenomatous crypt pattern!
■ Hamartomatous polyp
When in doubt, it's hamartomatous !
■ Malignant polyp
The crypt pattern is too organised and regular for cancer!
explanation
Sadly, an intimate knowledge of the Kudo crypt patterns doesn't help you here! Adenomatous polyps should be covered with one of the following;
In this case, the closest match is of a serrated polyp, which have wide open crypts with a somewhat jagged outline. However, apart from the crypt openings, there is nothing on this which looks like a serrated polyp! If you can't see a crypt pattern, the lesion is likely to be malignant. Conversely, if the lesion does have crypts but still doesn't look familiar, its either a hamartomatous polyp or, perhaps less likely, a 'Traditional Serrated Adenoma'. This lesion turned out to be a hamartomatous polyp. Why a 60 year old man would grow a hamartomatous colonic polyp remains a mystery ! |
Categories
All
|