|
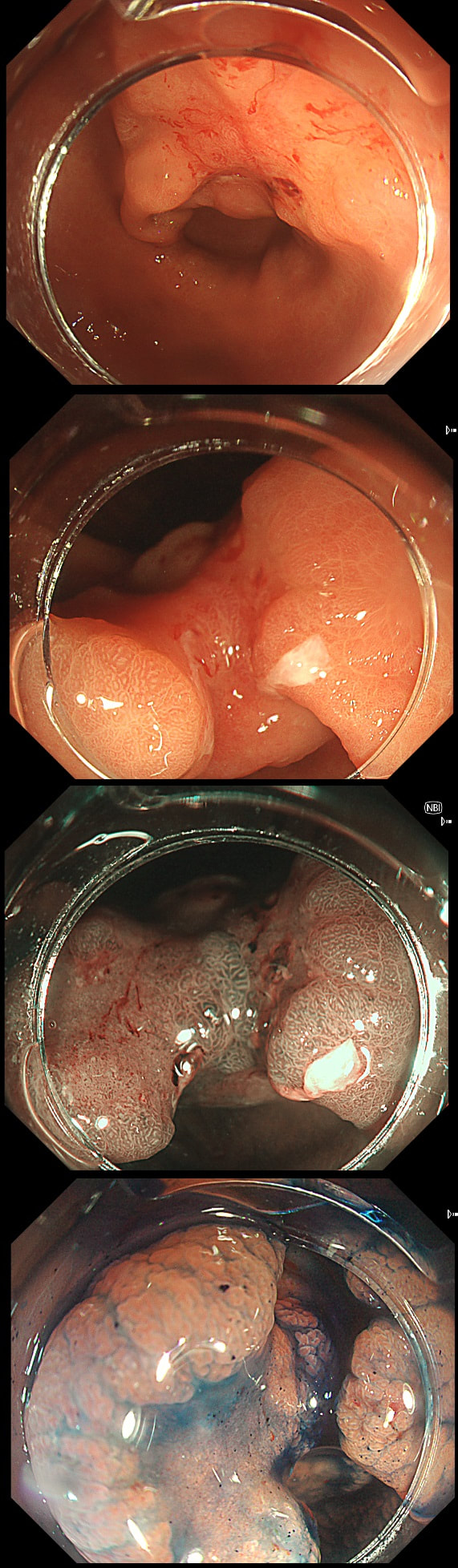
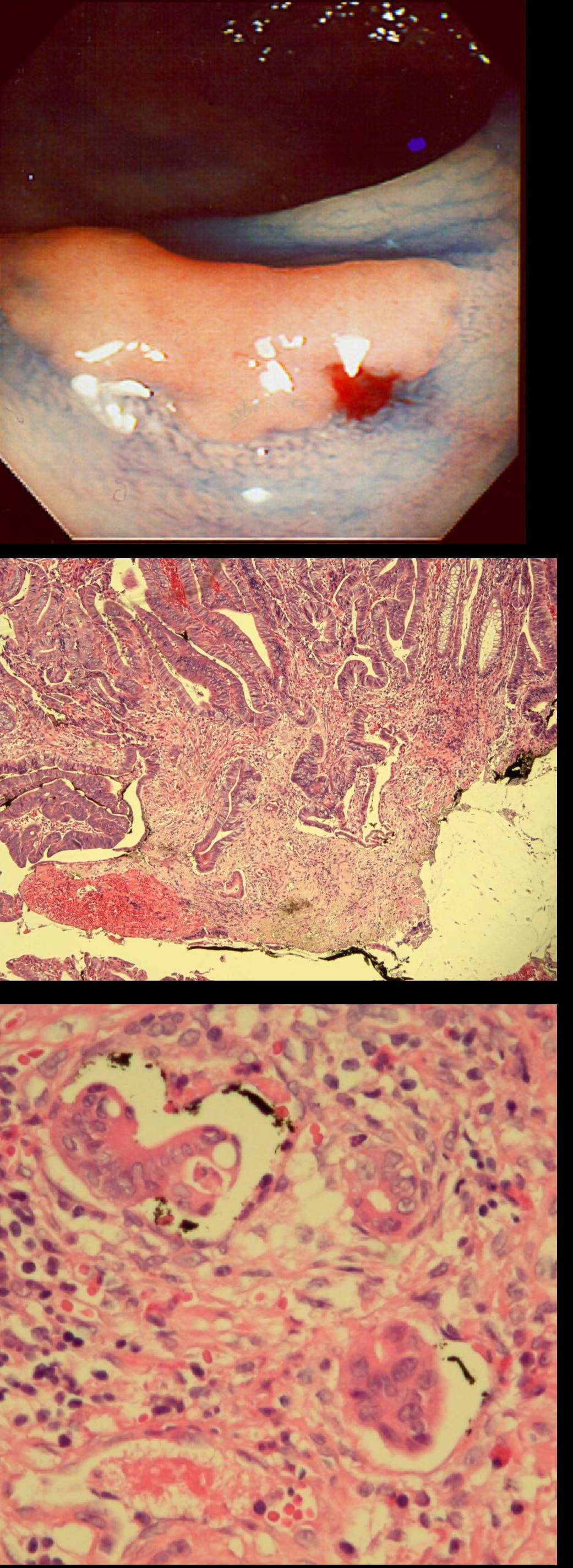
This gastric lesion is found in an 85 yr old man. Samples have confirmed a moderately differentiated gastric cancer but the lesion can't be seen on CT. The patient is frail with multiple comorbidities and declines both an endoscopic resection and surgery. However, he does ask how long it will take before he dies from the cancer?
WHAT WILL YOU ANSWER?
a) 1-2 yrs
Definitely too pessimistic!
b) 2-3 yrs
Hmm, perhaps if the lesion is poorly differentiated but it doesn't look it!
c) 3-4 yrs
This is probably how long it will before that cancer is at an advanced stage but patient will probably survive for another couple of years beyond that 'transition point'
d) 4-5 yrs
Probably too pessimistic still!
e) >5 yrs
With limited information this is the only figure backed up by some data.
Explanation
This question is something of an homage to Sir David Cox who developed the well known 'Cox Regression analysis'. Professor Cox died in 2022. As you know, the 'Cox Regression analysis' basically explores the relationship between the length of survival and more than one variable.
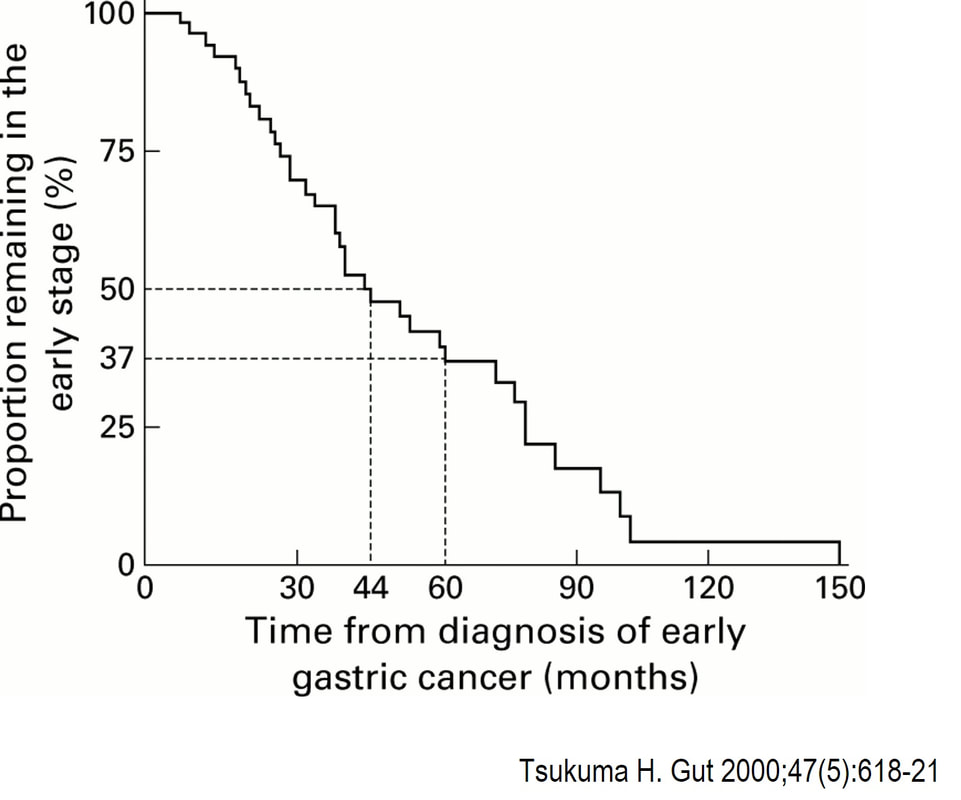
You would expect the following 'variables' to influence cancer-specific survival; histology, size of lesion, stage of lesion and perhaps the macroscopical type of early gastric cancer. Perhaps the location within the stomach also influences survival. There is some data that lesions at the gastric cardia are worse. Of course we don't have data on any of these 'variables'. However, I know of one paper by Tsukuma et al [Gut 2000;47(5):618-21] which did look at the natural history of 56 early gastric cancers in Osaka, Japan. This paper was actually a riposte to an earlier editorial from Leeds by one of my colleagues arguying that EGC is a 'pseudo-disease [Everett S. Lancet 1998;351:1350–2]. As you would expect, 56 EGC's is not enough for any sub-analysis of the above variables. Also they didn't actually look at survival. They looked at the average time it took lesion to progress from what appeared to be 'early' gastric cancer (i.e. confined to the mucosa or submucosa) as diagnosed by endoscopy. And here we reach the first issue! How sure can we be that the lesion in the image is an EGC and hasn't started to invade into the muscle propria (thus being advanced)? The lesion is about 2cm across and there is a an organised crypt pattern in the centre (can't comment on the vessel pattern as there are no magnification images). It does look 'early' and I stand at least an 80% chance of being correct in that diagnosis. Incidentally, 'lifting' is not a reliable sign in the stomach as any ulceration of the mucosa will tether it down, regardless of stage of invasion. The authors were pleased to report that if patients are left for long enough, those EGC's will grow and progress. No surprise there then! But the rate of progression was slow!!! The median time it took for 'early' gastric cancers to progress to advanced gastric cancers (i.e. T2 or worse) was estimated to be 44 months but probably shorter in patients with poorly differentiated cancers. Furthermore, the cumulative 5 year corrected survival in patients who never underwent surgery was about 63%. The answer to the question is therefore 'e'. This patient is likely to live more than 5 years from diagnosis of his early gastric cancer. Better than expected here is the Kaplan-Meyer curve below ! This scary looking polyp was found in the sigmoid in a patient on a polyp surveillance programme. WHAT IS THE LIKELY DIAGNOSIS?
a) Mucosal prolapse
Yes! You can't see any typical gland openings at the tip of this polyp. It's a mucosal prolapse polyp with a serrated lesion at the tip.
b) Adenomatous polyp
It's a battered polyp but without any recognisable crypt openings at the tip and therefore NOT adenomatous.
c) Malignant polyp
Well it looks scary and without an organised crypt pattern. All hallmarks of a malignant polyp. However, this lacks crypts because it's an innocent mucosal prolapse polyp!
Explanation
You've seen a few examples now of the 'mucosal prolapsing conditions' which include solitary rectal ulcer syndrome and inflammatory cloacogenic polyps. First described in 1985 [GIE 1985;31:196–9] they are usually said to be rare. However, experienced endoscopists know that these are common in the sigmoid. I've never seen a mucosal prolapse polyp outside of the sigmoid and would actually not make this diagnosis elsewhere in the colon. Histology is usually said to be the way to "confirm the diagnosis and rule out cancer". However, for me it's an endoscopic diagnosis. But I do understand that these lesions do look alarming. For this reason, I sometimes take it upon myself to resect the tip of the lesion, proving to all those of little faith, that the lesion is absolutely innocent. However, I'm not sure that my surgical colleauges are always reassured by the histology which usually rambles on about 'distorted elongated branched crypts, with fibromuscular obliteration of lamina propria with lots of intramucosal haemorrhages and a splayed and hypertrophied muscularis mucosae". However, the bottom line of that pathology report will read; 'No dysplasia'. This patient is undergoing a gastroscopy because of dyspepsia. WHAT IS THE LIKELY DIAGNOSIS?
a) Hyperplastic polyp
It's unusual to see a ulceration on a hyperplastic polyp
b) GIST
Unlikely as the lesion isn't covered with entirely normal looking gastric mucosa
c) NET
Although there are none of the classical chunky vessels around its neck, there are other smaller nearby lesions with some unusually large vessels
d) Adenoma
Gastric adenomas are usually flat, plaque-like lesions
e) Early gastric cancer (EGC)
EGC is a good guess and patients with atrophic gastritis often do have nearby small type I gastric NET's. However, this isn't an EGC.
explanation
As you well know, gastric NET's are classified as; type I (70%-80% of gastric NET's) linked with hypergastrinaemia secondary to an atrophic gastritis and classically appearing as multiple, small gastric nodules. Then we have the rare type II gastric NET which account for about 5-8% is associated with hypergastrinaemia from a gastrin-secreting tumour such as in the MEN-1 syndrome or the Zollinger–Ellison syndrome. This was a type II gastric NET which has arisen in a patient with a pancreatic gastrinoma and MEN. Several other much smaller NET's have arisen in the nearby gastric mucosa. Finally, we have the type III NET (20%) which are solitary, large nodules with a high mitotic index arising in a healthy gastric mucosa. These are the ones not to miss as they need a cancer-like gastric resection. To remind you of the lessons from Prof Mark Pritchard's Podcast on gastric NET's, you should; AT ENDOSCOPY: • Look for atrophic gastritis • Consider using some pH indicator strips to measure the gastric pH (unless pt is taking PPI) • Identify all the NETs, record their size and number and sample them for histology and grading • Take antral and corpus biopsies and ask pathologist to do report on the presence/absence of gastric atrophy and intestinal metaplasia and also ask them to carry out immunohistochemistry stains for ‘gastrin’ in the antral biopsies and ‘chromogranin’ and ‘synaptophysin’ in the gastric body samples. • Look into the second part of the duodenum for the small submucosal gastrinomas which occasionally are seen in MEN-I • Consider samples for Coeliac disease if the patient has IDA CONSIDER OFFERING ENDOSCOPIC RESECTION FOR: • type I gastric NETs if >10-15mm • type II gastric NET if they’re causing problems (eg bleeding) and/or gastrinoma can’t be resected • type III gastric NET <1cm (provided that it's no worse grade 1/low grade 2 !) HISTOLOGY: If that proliferative index comes back surprisingly high (>10%), make sure that the pathologist hasn't inadvertently counted Ki67 positive cells in the nearby gastric mucosa. Atrophic gastric mucosa is usually more proliferative than the NETs! BLOOD TESTS: • FBC • Full haematinic screen including B12 and Ferritin of course • TFTs • Anti-parietal cell AB & Intrinsic factor AB titres • Serum gastrin level • Chromogranin level • Calcium and PTH level (particularly if MEN1 is suspected) REQUEST THE FOLLOWING SCANS FOR EVERYONE WITH LIKELY TYPE II AND III DISEASE: • CT • 68Gallium DOTA-peptide PET/CT scan • EUS to search for duodenal wall gastrinomas and small gastrinomas within the pancreas which CT can't see and to search for lymphadenopathy close to the NET
This was found in the duodenal cap of a patient undergoing gastroscopy because of dyspepsia.
APART FROM TAKING SAMPLES, WHAT ELSE SHOULD BE DONE?
a) take a CLO test
Absolutely correct!
b) take gastric biopsies
Will show a Hp associated gastritis
c) take samples from D2
That's a no from me...
d) request an EUS
Never seem to be the right answer!?
e) request a CT
Perhaps if bx against all odds with suggest lymphoma but that would be a long shot
explanation
You first guesses when you find funny bumps in the duodenal cap should be gastric metaplasia and gastric heterotopia. Brunner's gland hyperplasia is also common but are never multiple (at least I have never seen a case with more than one nodule from Brunner's gland hyperplasia).
This is a case of foveolar gastric metaplasia in the duodenal cap. Basically, the mucosa in the duodenal cap has become more 'stomach-like' with crypts lined by mucus secreting cells (foveolar cells) which are different to goblet cells found elsewhere in the GI tract. Gastric metaplasia is common in patients with a history of peptic ulcer disease and is thought to be a defence response or adaption to the presence of excess acid in the duodenum. It's thought that Helicobacter are able to colonise the foveolar gastric metaplasia in the duodenum and this contributes to duodenitis and duodenal ulceration. So, what should you do next? Do a CLO test for Helicobacters of course! This lesion, situated in a very spastic sigmoid, has been referred for resection. WHAT IS THE LIKELY DIAGNOSIS?
a) lesion is not neoplastic
Yes, hard to believe but true!
b) lesion is adenomatous
Lesion is 'battered' but crypts don't look typically adenomatous
c) lesion is malignant
No way, lesion isn't angry red, chunky or devoid of crypts!
explanation
I couldn't stop myself! After yesterday's case of a rectal 'prolapse polyp, part of the 'Mucosal Prolapse Syndrome', I had to show an example of a sigmoid mucosal 'traction polyp' (my nomenclature). The mucosa at the apex of this sigmoid fold is traumatised and inflamed but not actually adenomatous! Histologically these lesions also appear somewhat bashed up. This is where pathologists may see 'pseudo-invasion' which is actually movement of crypts due to trauma and inflammation. The sigmoid colon is the most 'powerful' part of the colon developing the force needed to go to the toilet. Presumably this is the reason that diverticular disease first develop in the sigmoid. The force can also create these pseudo-polyps from patches of inflammation which I presume gets tugged along with each peristaltic wave. The end result is that this is the most difficult part of the colon to make head an tail of polyps. These are common lesions and if you are a 'therapeutic endoscopist', you will be refer these lesions. In these cases, I don't go overboard by placing a snare far down the 'pseudo-stalk'. If you did, you will find that it's taking a long time to cut through all that healthy sigmoid mucosal fold and you run the risk of a perforation (early or late). Instead, I just catch the tip of the fold and ask my assistant to close the snare as quickly as possible. Of course, you don't need to worry about a type of chunky central vessel which you may find in an adenomatous polyp. Analysis of a small piece of mucosal apex confirmed a normal mucosa. Hopefully this was enough for everyone to relax ...
A polyp found in the descending colon and removed as a single fragment (H&E attached)
WHAT IS THE DIAGNOSIS
a) Tubular adenoma
Yes but you are only partly correct!
b) Tubulovillous adenoma
No way, this is a LST-NG!!!
c) Villous adenoma
Absolutely not - there are no villi!!!
d) Sessile serrated lesion
Looks like it but histology doesn't!
e) Malignant polyp
Full marks!
explanation
This is a LST-NG type of lesion (laterally spreading tumour of the non-granular type). They are always TA's (tubular adenomas) and often (but usually not), harbour HGD or cancer. I guess that we can't really be sure about the crypt pattern as this is a non-magnified image. However, looking at the histology slide with narrow crypts, I expect that the crypt pattern is probably IIIs (small round crypts) which goes with TA+HGD. Must admit that I was surprised to find invasive cancer and LVI (lymphovascular invasion) in a small lesion such as this! The last image shows clusters of malignant cells within lymphatics. Of all the 'markers' to suggest that the patient needs surgery, LVI is the most important!
This is the scar following the piecemeal removal of a sigmoid polyp some 6 months ago. It was a 15mm tubular adenoma harbouring high-grade dysplasia. Samples from the EMR scar has showed 'distorted glands' only.
WHAT WOULD BE THE CORRECT FOLLOW UP?
a) organise an immediate follow-up
WTF ! This not a normal scar! You absolutely need to organise more samples, and perhaps a CT !!!
b) organise a follow-up in 6 months time
Would have been a mistake !
c) organise a follow-up in 12 months time
Would have been a big mistake!!!
d) organise a follow-up in 3 years time
May have cost the patient his life!!!
explanation
The first EMR was piecemeal and histology could of course not confirm that the resection had been complete. Indeed the 'index histology' reads oddly mentioning "frequent mitotic figures" and "back to back glands". To a gastroenterologist these words does not sound particularly alarming.
However, the pathologist was trying to say "this looks like cancer but I can't actually make that diagnosis" !!! Indeed this doesn't look like a normal EMR scar! The whole area is indurated as if there is an infiltrative process below the mucosa. Histology was reassuring mentioning some distorted crypts only. Sadly, the endoscopist was content with the reassuring repeat histology and did not reflect on the worrying endoscopic appearances. He did NOT organise a second round of post-EMR samples and the patient returned 2 years later with an advanced cancer. The take home messages from this sad story?
A 21-year-old man presents with a past history of intermittent abdominal pains. For the last year he has suffered with more frequently bouts of abdominal pains and more recently he has started to vomit some 30 minutes after eating. He has been started on lansoprazole for a couple of months with no response. At gastroscopy (photograph) there is a severe gastritis and a tight pylorus requiring dilatation to examine the duodenum (which was unremarkable).
His blood results are as follows: Hb 11.2 g/l MCV 105 fl WCC 11.9 x 109 Plat 395 x 109 Basal gastrin 180 pg/ml (<75) WHAT IS THE MOST LIKELY DIAGNOSIS?
a) Hp associated gastritis
Doesn't explain all the 'issues' !
b) Zollinger-Ellison syndrome
There is a simple explanation to that elevated gastrin level!
c) Crohn's disease
Well done!
d) Gastric lymphoma
The antrum would do but how do you explain the macrocytosis?
e) Diffuse type gastric cancer
Could look like this but he is a bit young isn't he? !
explanation
Although the patient has a raised gastric level, this can be explained by the lanzoprazole. The macrocytosis is more difficult to explain. An autoimmune gastritis would be expected to be worse in the gastric body than in the antrum. In fact, the only other possibility is that the patient has terminal ileal Crohn's disease, causing malabsorption of B12 as well as a Crohn's gastritis. This was indeed the case! Actually, the diagnosis had already been confirmed by antral biopsies before I carried out the dilatation! Wouldn't like to dilate a diffuse type cancer !
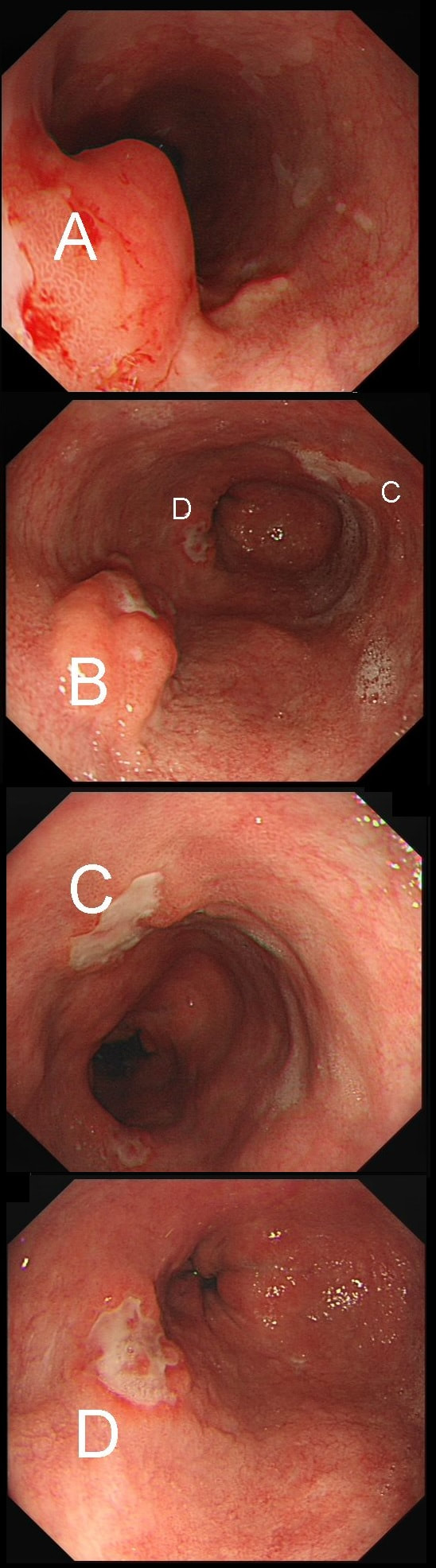
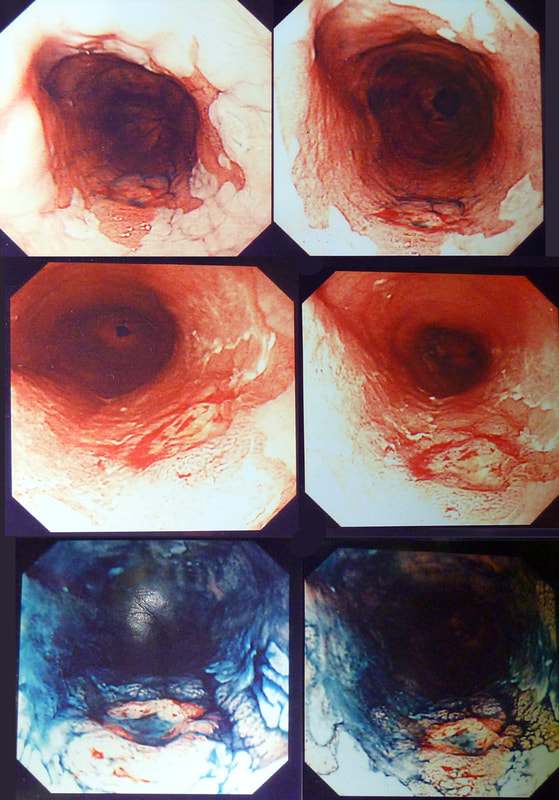
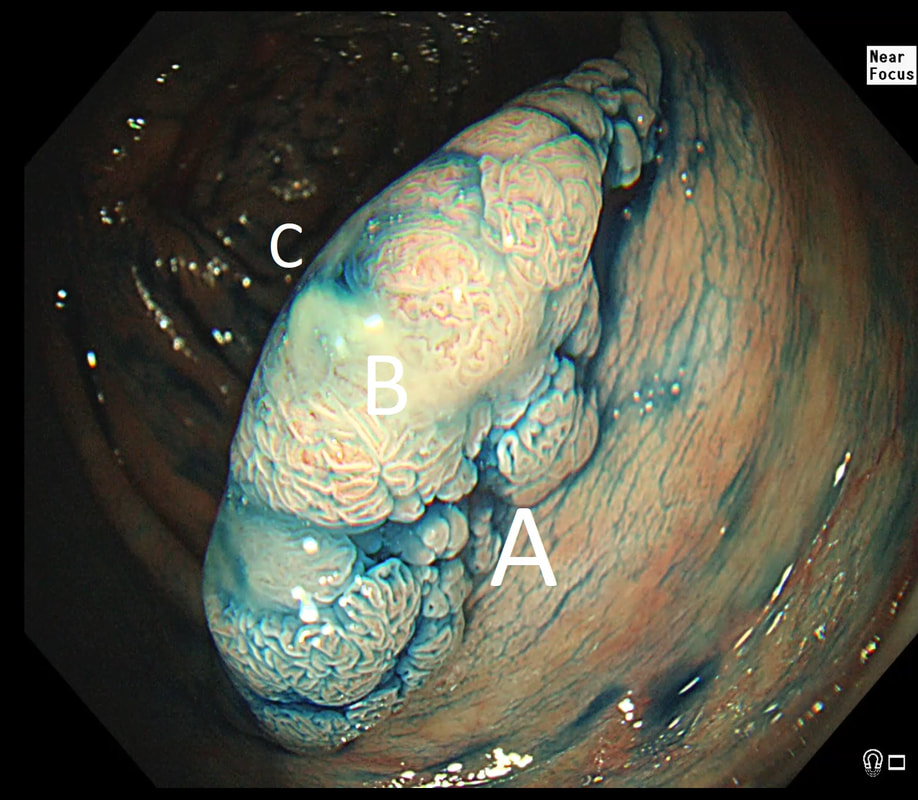
55 yr pt with a Barrett's nodule is referred for an endoscopic resection. I'm surprised to find 4 abnormalities within his 10cm stretch of Barrett's
WHICH OF THESE IS THE MOST LIKELY TO BE ENDOSCOPICALLY RESECTABLE?
a) Lesion A
I agree! Of these 4, A is the only one which isn't ulcerated and therefore is least likely to be invading deeply.
b) Lesion B
Would be my second guess as the ulcerated area seem superficial
c) Lesion C
Don't like the ulceration!
d) Lesion D
Would be my least favourite lesion to attack as the ulceration suggests deeper invasion and poor lift into my cap.
explanation
This may be something of a record, 4 synchronous lesions! Clearly A, B and D are malignant. At first, ulcer C seem more innocent without an elevated edge but on closer assessment, it also has a slightly elevated rim.
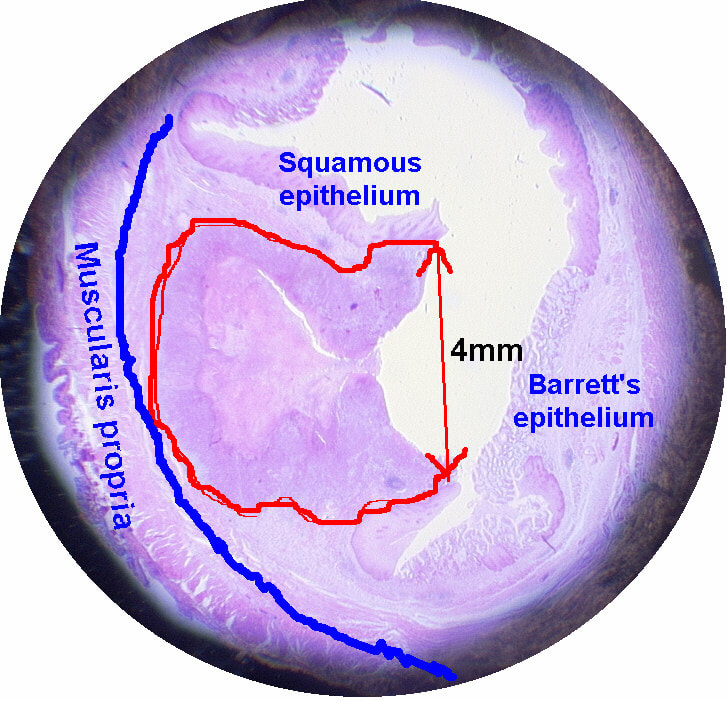
Of these 4, A is the only one which isn't ulcerated and therefore is least likely to be invading deeply. Of course, they are assessed together as there is no point in EMR'ing one only. Either all are curable endoscopic means or none are ! Rather than going ahead with attempting to resect these, I actually bailed out and took samples from each lesion. Biopsies showed invasive, poorly differentiated adenocarcinoma at each location! Clearly, this patient has multifocal 'bad disease' which endoscopy is unlikely to cure in my opinion. I believe that surgery is a far better option and the patient is currently awaiting his oesophagectomy. If you still are not convinced of the pitfalls in trying to deal with ulcerated Barrett's lesions, have a look at the lesion below. Two rounds of sampling had indicated that the lesion harboured HGD. However, I failed to remove the lesion and ultimately the patient underwent an 'Ivor-Lewis'. You can see the histology yourself. The 4mm surface is literally the tip of the iceberg and below you can see the cancer (red line) invading up to the muscle propria.
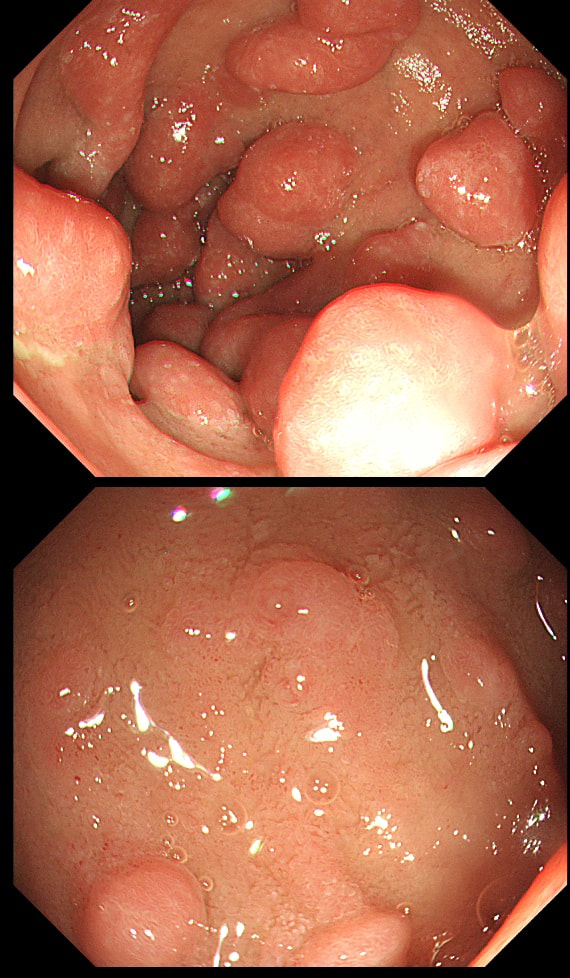
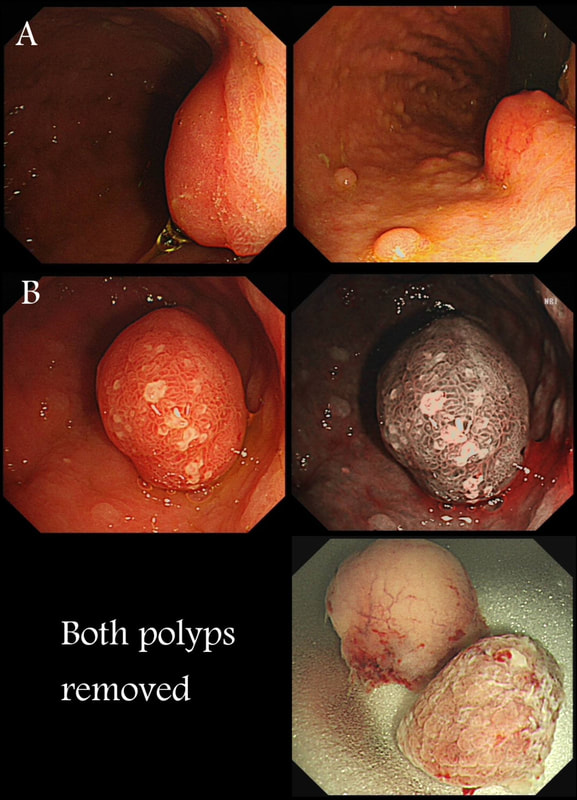
Two gastric polyps (labelled A & B) were both removed from the stomach of a 60 year old woman who complained of indigestion.
WHAT IS THE LIKELY DIAGNOSIS?
■ Both are hyperplastic
But the look different to each other!
■ NET + HP polyp
Well done ! And presumably you know which is which?
■ Both are NET's
But they look different!
■ HP + Adenoma
How about those fine vessels seen in the second image?
■ Both are adenomatous
But they look different!
explanation
You can see the thin vessels crawling up the side of polyp A. This appearance is typical of a gastric NET. Polyp B has a more villous surface with a few white spots - all typical of a hyperplastic gastric polyp. Some of these may contain some malignant cells which somehow generate a hyperplastic/reactive/inflammatory reaction around themselves. However, most hyperplastic gastric polyps are benign and arise secondary to a Helicobacter pylori gastritis. For this reason, you should always do a CLO test in these cases.
Every few weeks, I look up the notes on Prof Pritchard's' Podcast on gastric NET's to remind myself of the workup of these cases. As you remember, you should take samples to confirm an atrophic gastritis whenever you find a gastric NET. In this case, the patient did NOT have an atrophic gastritis. Instead, there was a Hp associated gastritis which is the reason for the second polyp. We realised that what we had was a TYPE II gastric NET! Analysis confirmed that the 17mm was WHO grade II. The finding of anything else than an innocent Type I gastric NET means that further imaging was required. A little late in the day of course but fortunately, the following imaging investigations were unremarkable:
Anyway, below is a reminder of what to do at gastroscopy, when you have a case of gastric NET:
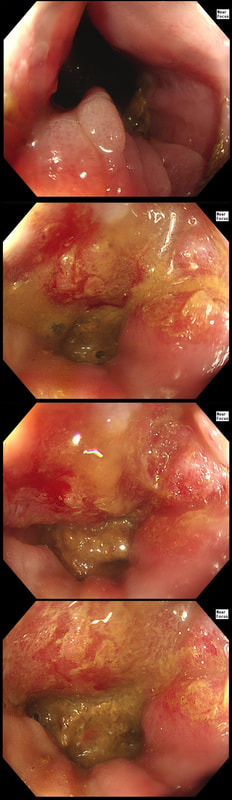
This patient was referred for a flexible sigmoidoscopy because of PR bleeding. However, the only abnormality found was a sore anal canal. Samples are taken of course.
HOW WOULD YOU NOW ADVICE THE PATIENT?
■ We'll see you in clinic once histology is to hand
But there is a degree of urgency here!
■ Symptoms may improve with 'anusol'
No they will not!
■ We will try topical mononitrate first
You are barking up the wrong tree!
■ We will organise a scan next
Yes, a rectal MRI revealed something important!
■ Inject 80mg of triamcolonone
You are missing the point!
explanation
Actually, this isn't a case of haemorrhoids or an anal fissure. Histology reported; " Within hyperkeratotic epidermis there are scattered individual highly atypical infiltrating malignant cells with frequent apoptosis and moderate clear cytoplasm. There is no ulceration or significant inflammation."
Actually, this is a case of Perianal Paget's disease, - a VERY rare condition!!! You'll remember that a 'puckering' of the skin around the breast areola is associated with underlying breast cancer. This was first reported by Sir James Paget in 1874. However, a few years later, the same phenomenon was described elsewhere, so called "extramammary Paget's disease". In descending order of frequency, this has been described at; the skin of the vulva, perineal skin, perianal skin and the skin of the scrotum. Paget's disease, is usually NOT a primary cancer of the apocrine glands of the skin. It's almost always secondary to a nearby cancer of the rectum, anus or prostate. In this particular case, further imaging revealed a nearby prostate cancer! ! This patient attended for dilatation of his biopsy confirmed peptic oesophageal stricture. Clip has been speeded up somewhat. WHAT WOULD YOU ORGANISE NEXT?
■ A clinic appointment
To ask pt about the swallowing? Hmm, somethings missing!
■ Another dilatation in 2 weeks time
Stricture not very tight and the dilatation should last longer
■ an early follow up OGD for biopsies
The mid-oesophageal stricture is peptic but what of the mucosa below?
explanation
The background to this case is a recent audit which we did in Leeds on missed upper GI neoplasia. We have had several instances of oesophageal cancers being missed when the endoscopist focused too much on the 'task in hand'. For example, we have had several SCC's missed when the endoscopist was carrying out a Barrett's surveillance endoscopy. This is another example of a missed (intramucosal) adenocarcinoma, glimpsed about 10 seconds into the clip in the 3 O'clock position. With mid-oesophageal peptic strictures there is often a stretch of Barrett's below which of course must be assessed and sampled at the earliest convenience. Of course, one can argue that when the job is to do something therapeutic, such as placing a PEG or removing a large polyp, it is 'permissible' to miss a cancer elsewhere. After all, the objective is not to undertake a careful diagnostic examination but to 'do a job'! I'm a strong believer that 'diagnostic' examinations and 'therapeutic' examinations must be clearly separated when you are looking at 'missed lesions. There is a distinct 'therapeutic window' during all endoscopic procedures done without a general anaesthetic. In the upper GI tract, it's up to 20-30 minutes and in the colon I think that it's up to 45-60 minutes. Your patient will not thank you for wasting the valuable minutes of your therapeutic window on carrying out a full diagnostic examination. Of course, after your therapeutic procedure you should consider if an early diagnostic gastroscopy or colonoscopy is needed. But carrying out a full assessment of the squamous portion of the oesophagus at the time of a Barrett's surveillance examination doesn't add much to the procedure. Similarly, when a mid-oesophageal peptic stricture is found, one should realise that the reason that the peptic stricture is in the middle of the oesophagus and not the gastro-oesophageal junction, is probably that there is a a Barrett's segment below the stricture. It doesn't add much time to the procedure and that IMca could have been spotted earlier! This is an elderly patient undergoing an OGD because of IDA (iron deficiency anaemia). A lesion catches my eye on the anterior gastric wall WHAT IS THE LIKELY HISTOLOGY?
■ Gastric xanthelasma
The colour is suggestive and gastric atrophy would be the link but it's not quite right...
■ Healed GU scar
But how about that funny crypt pattern?
■ Gastric adenoma
Yes, doesn't this look like a tubular adenoma?
■ Early gastric cancer
Wouldn't the crypt pattern then be very disorganised or perhaps very small?
explanation
The pale colour is odd and reminiscent of gastric xanthelasma which as you know is linked with gastric atrophy which is the likely cause of this patients IDA. However, when I zoom in on the area, the crypt pattern is different here. Of course, this does not fit with a xanthelasma or a scar from a healed gastric ulcer (GU) either for that matter. Interestingly, almost everyone thought that this was an EGC. However, THERE IS a distinct crypt pattern in the centre of the lesion. Furthermore, the lesion isn't red. Remember that cancers encourage the growth of small irregular capillaries which gives them a red colouration. Finally, it doesn't have the typical flat-elevated with a central depression (IIa+IIc) growth morphology. Therefore, your first guess should be a gastric adenoma! This is actually a gastric tubular adenoma which we found in an elderly frail patient with atrophic gastritis some 10 years ago. As she had some comorbidities and it was only harbouring LGD, we decided to keep an eye on the lesion on a yearly basis. The risk of progression is supposedly only 5% with tubular adenomas in the stomach. In contrast, villous adenomas are much more likely to progress (40%). The BSG gastric polyp guidelines have the references if you want to look this up. Of course, the issue is not entirely clear-cut as risk of progression also increases with the size of the lesion (and this is probably 2cm in size) and also with age (patient is now 86 yrs). In some ways, making an initial decision to either 'attack' or 'abort' would be easiest. After all, regular surveillance drains valuable resources and leaves you open to the possibility that at some point in the future, the patient is no longer a candidate for anything more invasive than a haircut but now the lesion under surveillance shows evidence of progression. Then your patient could well ask the legitimate question why you didn't go ahead when he was younger and fit enough but instead wasted his time with pointless surveillance?! My own preferred way to navigate this minefield is to openly discuss the three options with the patient;
I often wonder if its the personality type which dictates what patients prefer. Perhaps, those who think 'my glass is half empty' usually want to have their lesion resected immediately whilst people who regards their 'glass to be half full', prefer to hope for the best and get on with their lives? This is beautiful polyp, perched on a fold, was found at the junction between the caecum and the ascending colon. The video gives you a better idea of the size and extent of the lesion. WHERE WOULD YOU PLACE THE NEEDLE FOR THE BEST 'LIFT' ?
■ On the side facing you
A convenient choice but lift will not be optimal
■ Into the apex of the polyp
That would be my choice!
■ Just behind the lesion
Better than into the fold facing you
explanation
I have no qualms about injecting straight into the middle of lesion provided that I'm sure that it's benign. The lovely gyrate pattern of this polyp tells you that it's a TVA, likely to harbour no more than LGD. Injecting into the fold facing you is likely not to raise the 'blind side' of the polyp which extends down the back of the fold and onto the 'flat' beyond. Conversely, injecting into the back of the lesion would lift the back end but probably not the front. My choice was to inject into the apex (see video below) which resulted in a lovely lift. But, why not inject in two places? Because you should try to avoid injecting into more than one place (if it can be avoided). If you have made more than one hole in the epithelium you may will find that your injection leaks out through the previous hole and that the elevation is less effective. Of course when removing large, flat lesions multiple injection sites can't be avoide, unless you are removing the lesion by ESD of course.
|
Categories
All
|