|
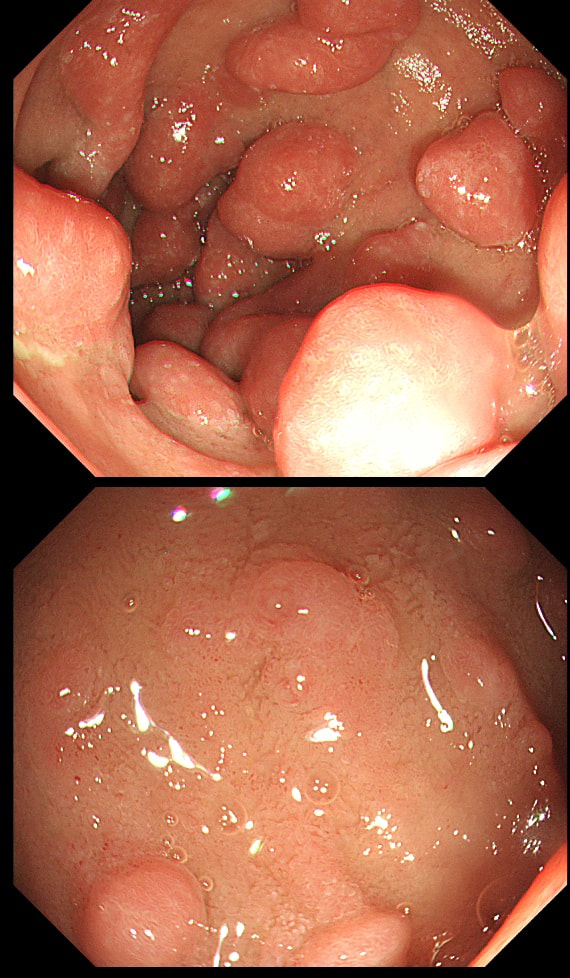
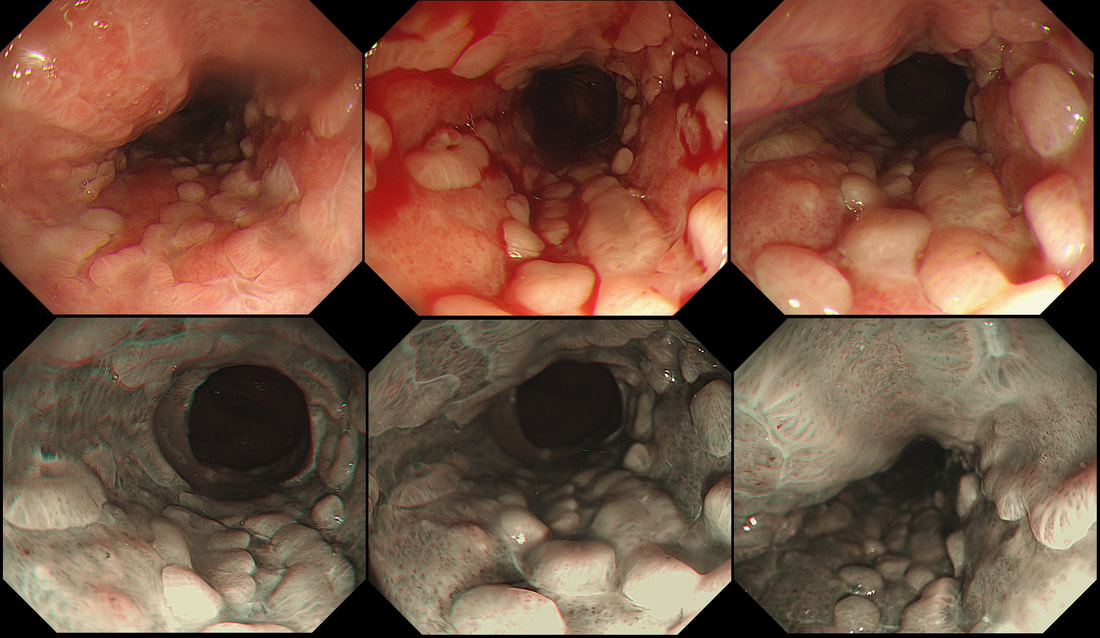
This was found in the duodenal cap of a patient undergoing gastroscopy because of dyspepsia.
APART FROM TAKING SAMPLES, WHAT ELSE SHOULD BE DONE?
a) take a CLO test
Absolutely correct!
b) take gastric biopsies
Will show a Hp associated gastritis
c) take samples from D2
That's a no from me...
d) request an EUS
Never seem to be the right answer!?
e) request a CT
Perhaps if bx against all odds with suggest lymphoma but that would be a long shot
explanation
You first guesses when you find funny bumps in the duodenal cap should be gastric metaplasia and gastric heterotopia. Brunner's gland hyperplasia is also common but are never multiple (at least I have never seen a case with more than one nodule from Brunner's gland hyperplasia).
This is a case of foveolar gastric metaplasia in the duodenal cap. Basically, the mucosa in the duodenal cap has become more 'stomach-like' with crypts lined by mucus secreting cells (foveolar cells) which are different to goblet cells found elsewhere in the GI tract. Gastric metaplasia is common in patients with a history of peptic ulcer disease and is thought to be a defence response or adaption to the presence of excess acid in the duodenum. It's thought that Helicobacter are able to colonise the foveolar gastric metaplasia in the duodenum and this contributes to duodenitis and duodenal ulceration. So, what should you do next? Do a CLO test for Helicobacters of course!
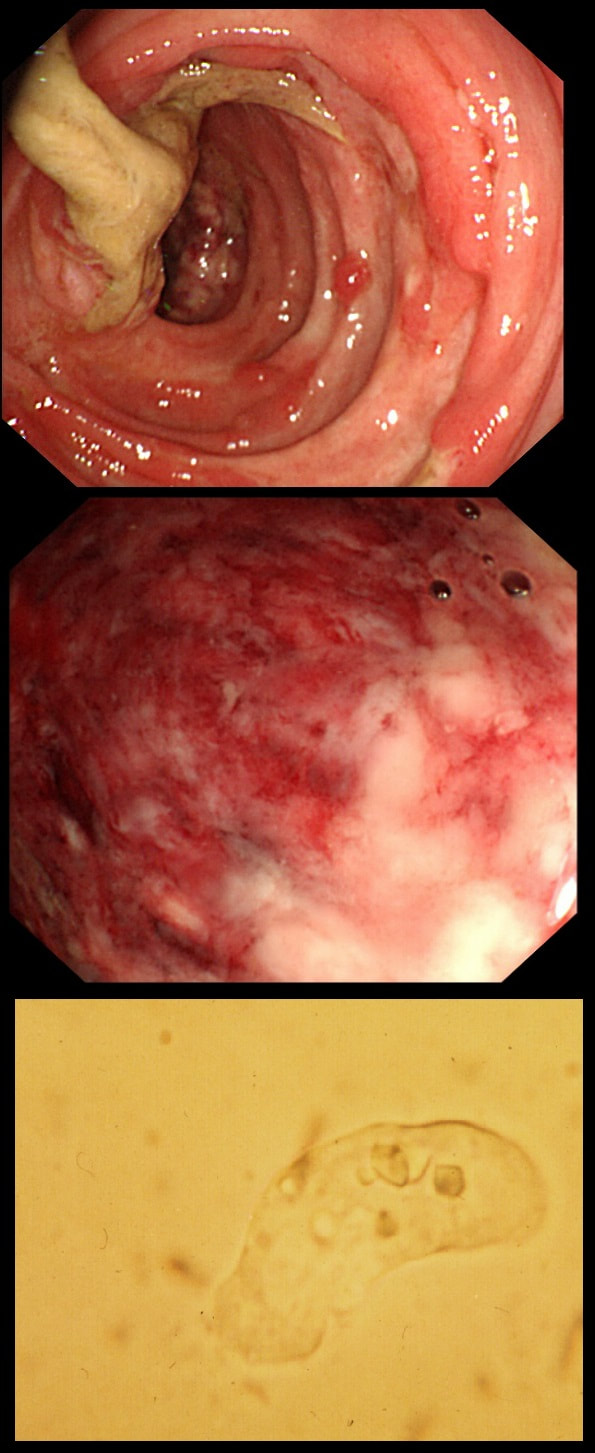
This patient developed bloody stool whilst on holiday in Africa. They continue on return home and he undergo a flexible sigmoidoscopy. Of course, stool samples are obtained (last image).
WHAT IS THE PATHOGEN?
a) Clostridium difficile
Can be seen moving down a microscope
b) Salmonella enterica
Can give bloody diarrhoea but its not it!
c) Entamoeba histiolytica
Rasberry stool, to be examined warm
d) Campylobacter jejuni
May also give bloody stool but that's not it
e) Vibrio cholerae
Hmm, rice-water stool...
explanation
Let me give you some more clues! Classically, there are 'flask-shaped' ulcers found on resectional histology in which the mucosal surface ulcer is rather narrow with a wider, necrotic submucosal component in which the amoeba multiply. Stools have been described as like "Raspberry jam" and when examined whilst still warm, something can be seen moving. Of course PCR is the best way of confirming the diagnosis.
This patient had amoebic dysentery! Mild cases can resolve without metronidazole. This is the sigmoid colon and rectum of a young patient who have just undergone bone marrow transplantation. WHAT IS THE LIKELY DIAGNOSIS?
■ Graft versus host disease
You don't get ulcers in GVHD!
■ Checkpoint inhibitor colitis
You DO get ulcers with these but they wouldn't be used!
■ Pseudomembranous colitis
Doesn't cause ulceration
■ CMV colitis
Absolutely !
■ Herpetic colitis
Hmm, does this actually happen?!
explanation
In GVHD there are minimal mucosal changes whilst there is extensive ulceration in this case. You do get nasty mucosal ulceration with checkpoint inhibitors but these drugs would not be used in transplantation. C.diff doesnt' cause mucosal ulceration and the herpes virus does not infect columnar mucosa. This leaves CMV ! Cytomegalovirus is a herpes virus that infects the majority of humans. Primary infection in individuals with normal immune function is usually asymptomatic or result in mononucleosis-like syndrome (fever, lymphadenopathy, and atypical lymphocytosis on a blood film). After primary infection, CMV becomes latent in various host cells but are controlled by a functioning immune system. When re-activation occurs in patients with severely compromised immune function (transplant patients or patients with AIDS and CD4 count <50 cells/microlitre), uncontrolled CMV replication can ensue leading to fever, bone marrow suppression, and tissue-invasive disease, depending on where the reactivated cells are residing. Investigations includes serology, pp65 antigenaemia test, histopatholical analysis of any tissue obtained, and PCR based detection. Treatment of choice is oral valganciclovir or intravenous ganciclovir whilst IV foscarnet and cidofovir are second line agents.
This is the sigmoid mucosa of an elderly inpatient who has developed diarrhoea.
WHAT SINGLE TEST WOULD YOU RECOMMEND NEXT?
■ FBC
Of course it's not sensitive nor specific and does not exclude diagnosis if normal. Nevertheless, a very high WBC level is often found in fulminant pseudomembranous colitis.
■ Obtain a set of stool cultures
Probably not a priority and they should have done it already!
■ ELISA for C.diff toxin in the stool
Yes! Results available within a few hours with sensitivity of 65%-85% and specificity 95%-100%
■ Stool PCR for C.diff
Seems like a sensible investigation in patients with unexplained, new-onset diarrhoea but perhaps not the single most important test...
■ Abdominal X-ray
Important IF there is significant abdominal distension
explanation
Of course this is a case of Clostridium difficule associated colitis (pseudomembranous colitis). Patients usually present with diarrhoea, abdominal pain, and leukocytosis, and a history of recent antibiotic use. Other common symptoms include fever, abdominal tenderness, and distension.
In a symptomatic patient with typical colonic pseudomembranes such as in this case, arguably it would be sensible to recommend that treatment is immediately started. Stool testing should be considered in any patient with unexplained, new-onset diarrhoea (defined as 3 or more unformed stools in 24 hours in a patient not taking laxatives). Your local institution will probably have protocols for how patients should be investigated and all of the above test may well be part of the algorithm. When I was on the wards, toxin tests were favored over culture for diagnosis of C.diff because it was the toxins which mediate disease. detection of toxins was faster and correlated better with symptoms. However, there was a move towards 'molecular tests' (PCR for the bacterium) from 2009 because of concern that patients with C.diff could be missed by toxin tests. Of course, this raised the question; do toxin-negative patients with a positive C difficile PCR test result require treatment? Several studies have now indicated that about half of the patients with positive PCR test for C. diff do not experience adverse events without treatment and do not need treatment. For this reason, PCR testing for C.diff should not be used as the stand-alone diagnostic test. Instead it's patients with clinical disease (diarrhoea) AND a positive toxin assay who should be treated! There are lots of references for this statement and here is an open access article in JAMA: Treatment is to discontinue the responsible antibiotic and start therapy with oral vancomycin or fidaxomicin. Up to 50% of patients have a relapse after discontinuation of antibiotics, but most respond to a second course of therapy. In those who relapse again, faecal microbiota transplantation is very effective.
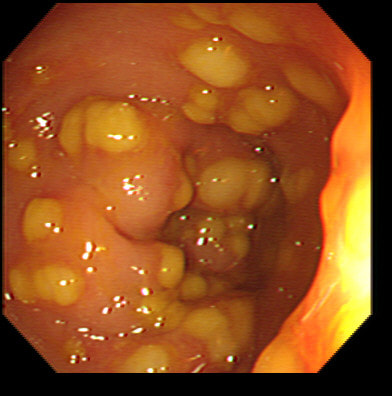
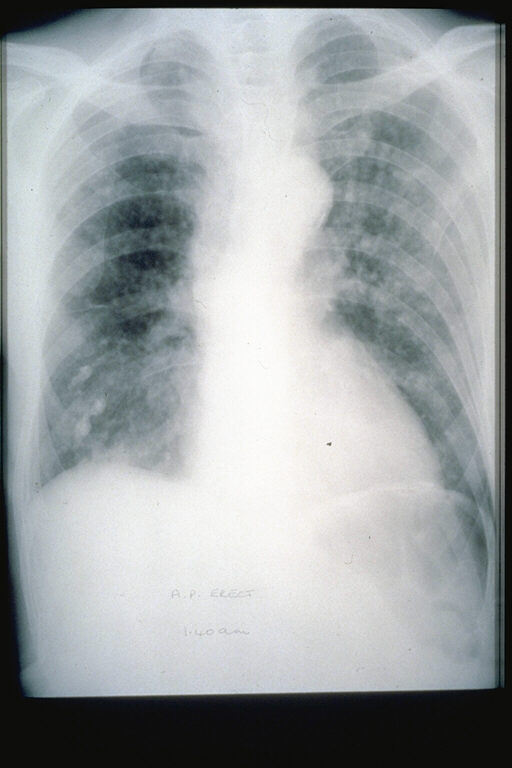
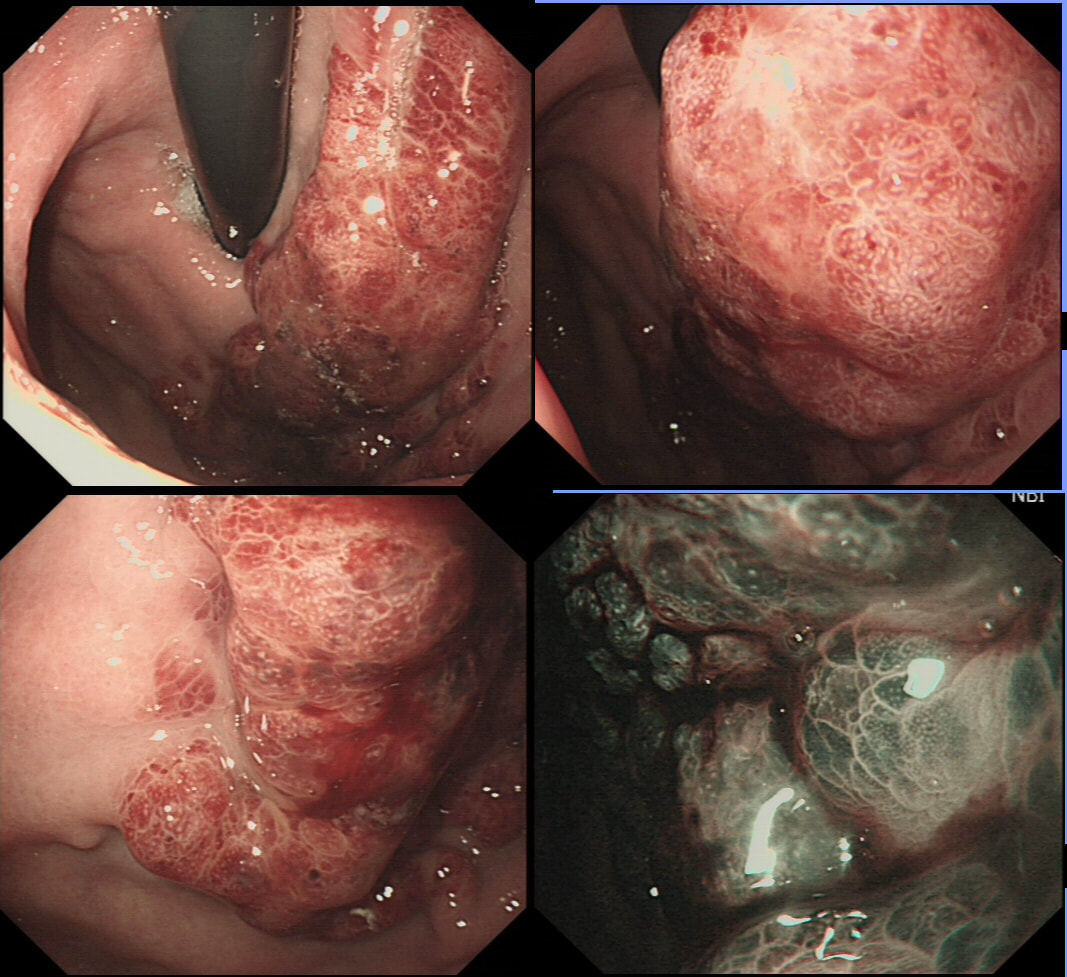
This odd looking thing was found in the gastric fundus of a middle age lady undergoing investigations for nights sweats, a dry cough and shortness of breath on exertion as well as weight loss (CXR enclosed).
WHAT IS THE LIKELY DIAGNOSIS?
■ CMV infection
INCORRECT!
■ Vascular malformation
I'm afraid not!
■ Gastric lymphoma
Unfortunately not!
■ Kaposis sarcoma
YES, you are right !
■ Gastric adenocarcinoma
INCORRECT
EXPLANATION
The patient had a two month history of night sweats, malaise, weight loss. More recently she developed a non-productive cough and shortness of breath on exertion. The CXR shows some diffuse interstitial infiltrates.
Of course, the patient has AIDS (the CD4 count was 3 only) and the chest signs is due to Pneumocystis carinii pneumonia. To be honest, when the patient attended for her OGD, the diagnosis of AIDS had actually already been made. The gastric lesion had also been sampled and the pathologists reported: "non-specific specialised and non-specialised gastric tissue showing a variety of changes including oedema and haemorrhage with haemosiderin laden macrophages, with areas of solid spindle cells, vascular areas with small channels, and extravasated red blood cells". As you've guessed, the pathologists were clueless. This was because whoever took the original samples had not told them that the patient had AIDS (!) I took a second set of samples and this time told them about the HIV infection. Of course, this is all they needed and promptly did immunohistochemistry for "Kaposi's sarcoma-associated herpesvirus" (also known as; human gammaherpesvirus 8 or HHV-8 virus). Infection with this virus is thought to be lifelong, but a healthy immune system will keep the virus in check. In the immunosuppressed, the virus somehow get patients monocytes to express 'anti-programmed cell death protein 1' (PD-1) on their cell membranes, which inhibits apoptosis and cause 'immune escape' in several tumours. The checkpoint inhibitors nivolumab and pembrolizumab block PD-1 and have an antitumor effect. You'll recognise these two drugs because of the awful colitis they can cause. This is the oesophagus of a 45 yr old woman 3 weeks into induction chemotherapy for AML when she develops retrosternal pain and fever. Treatment with meropenem, ambisome, itraconazole, aciclovir and intravenous omeprazole is started with improvement of symptoms. This is the lower third of her oesophagus. WHAT IS THE MOST LIKELY DIAGNOSIS? a) reflux oesophagitis b) infective oesophagitis c) squamous cell carcinoma d) adenocarcinoma e) infiltration by myeloid cells explanation
An unusual appearance to the squamous portion of the oesophagus! Lower down, the appearances were unremarkable and healing reflux oesophagitis was therefore unlikely. Stains for fungi and immunohistochemistry for CMV were both negative. However, there were some multinucleated squamous cells and after a long search by our histopathologist, a couple of likely viral inclusions were found. This is a resolving herpetic oesophagitis ! Resolving because the patient had already been on aciclovir for a week when the endoscopy was done. Small miracle that our pathologists found traces of the original infection! This is a common issue with immunosuppressed patients. The haematologists are VERY quick to start a broad range of anti-microbial medication, covering fungi, viruses and bacterial pathogens. By the time the endoscopy is done, the original pathogen is usually suppressed to undetectable levels. |
Categories
All
|