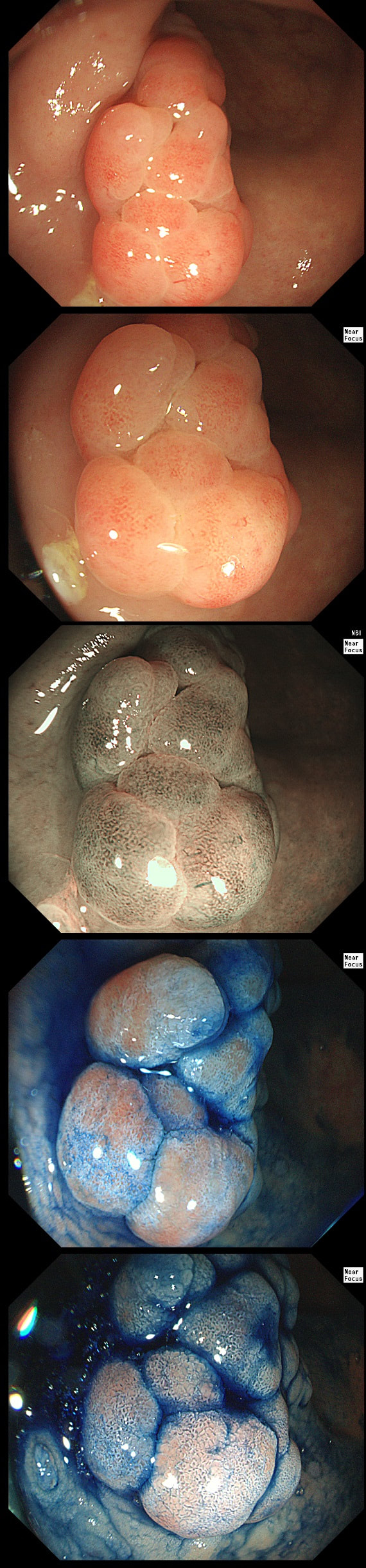
|
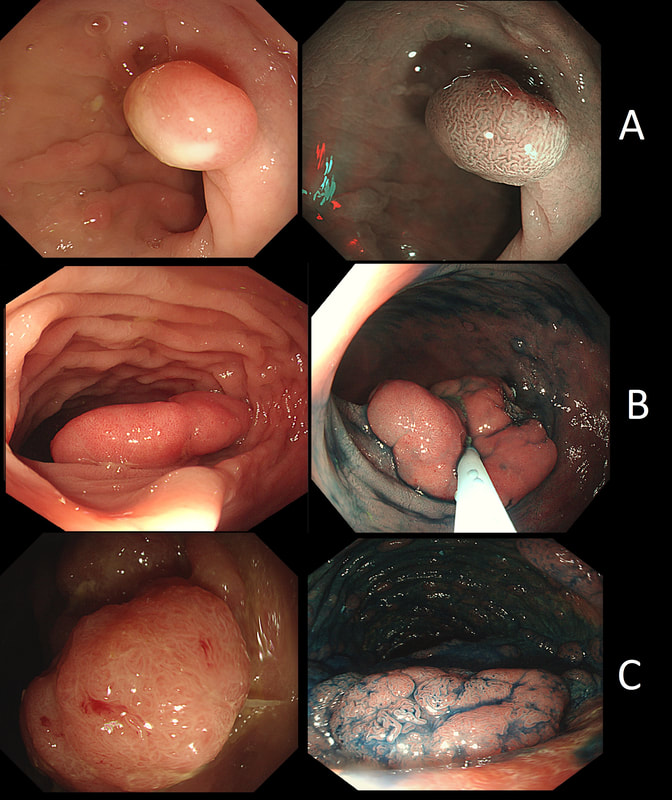
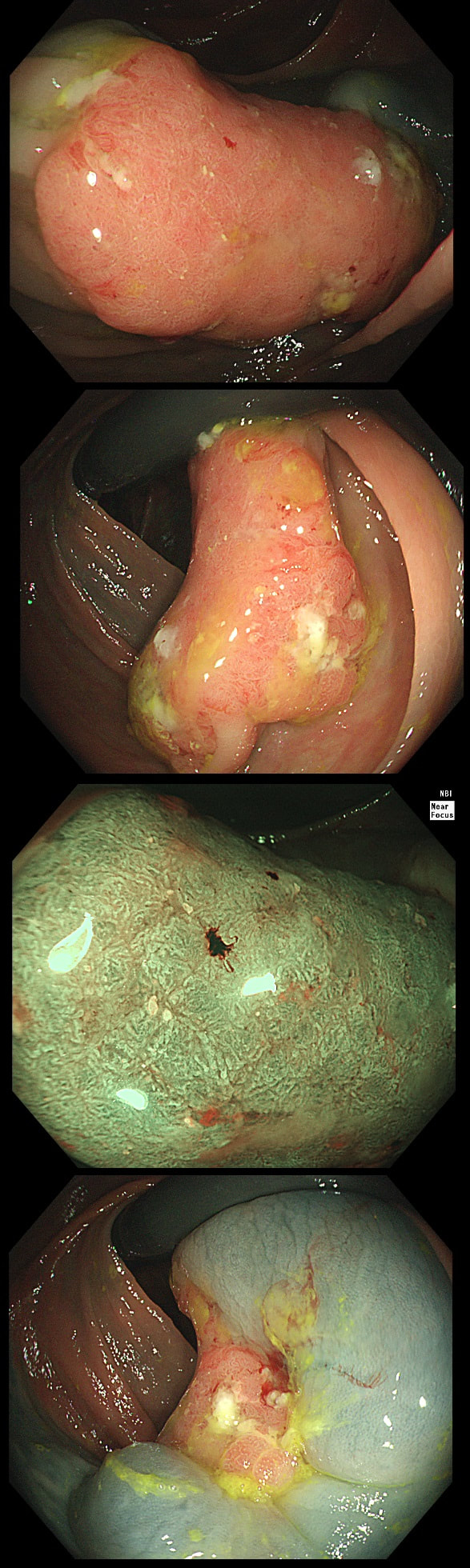
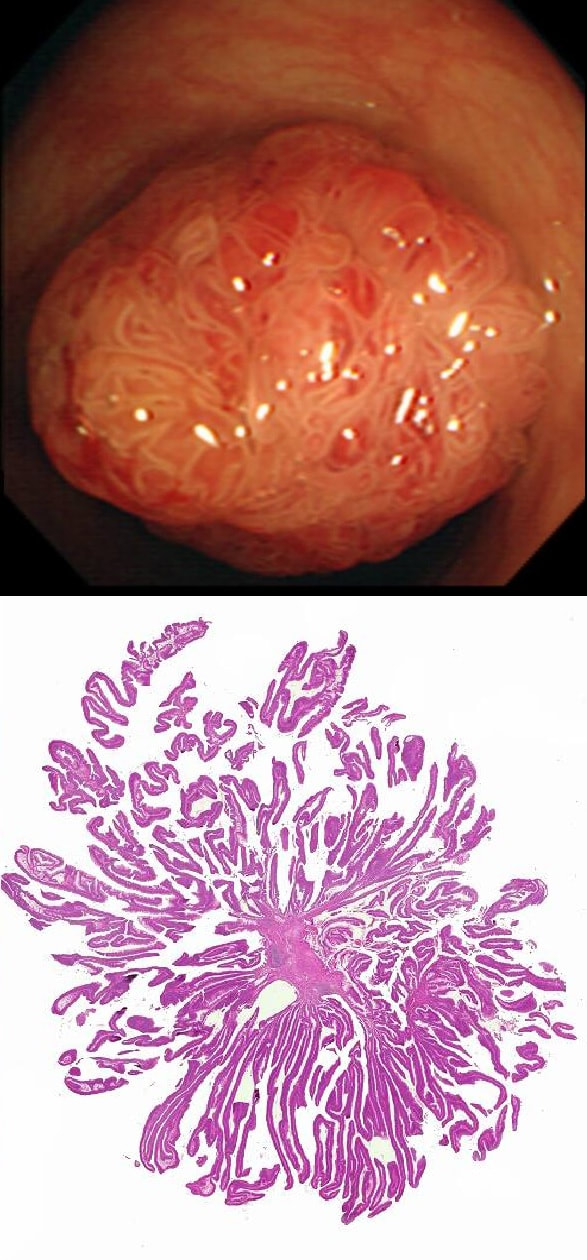
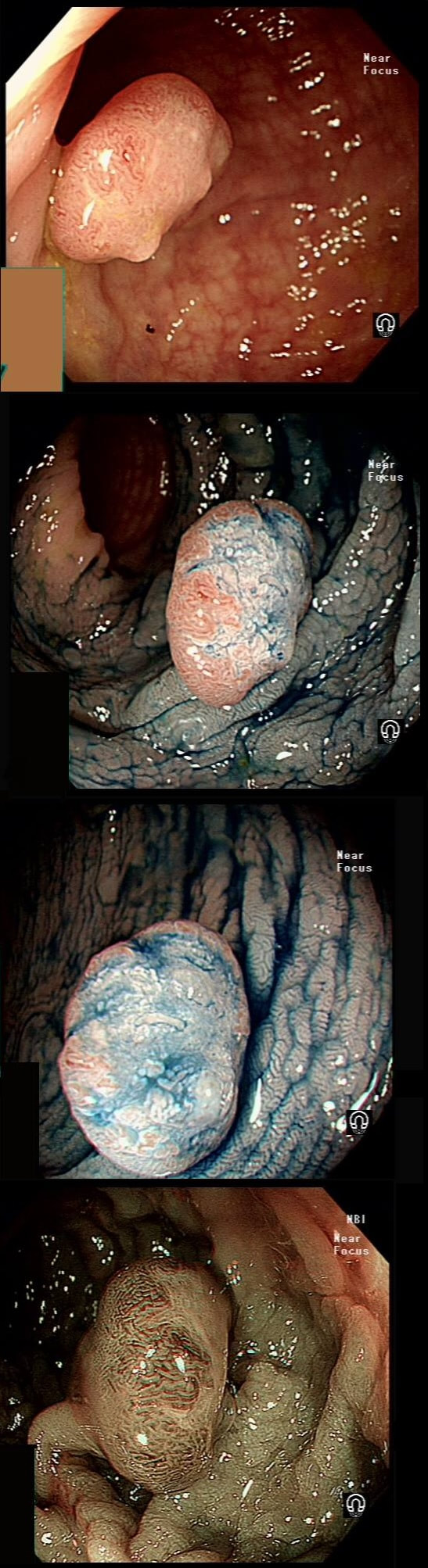
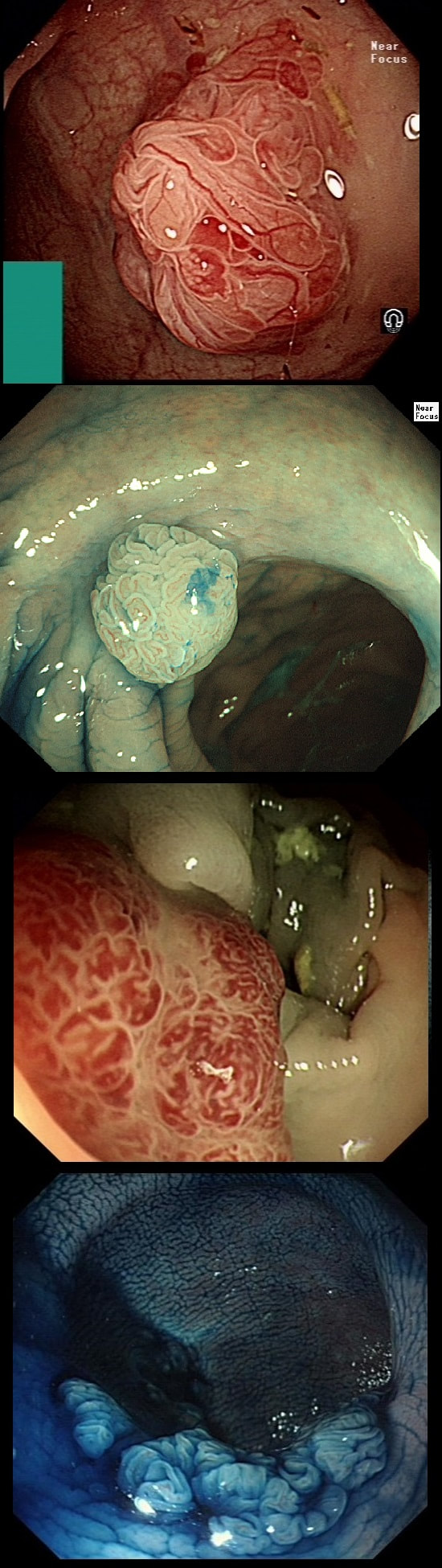
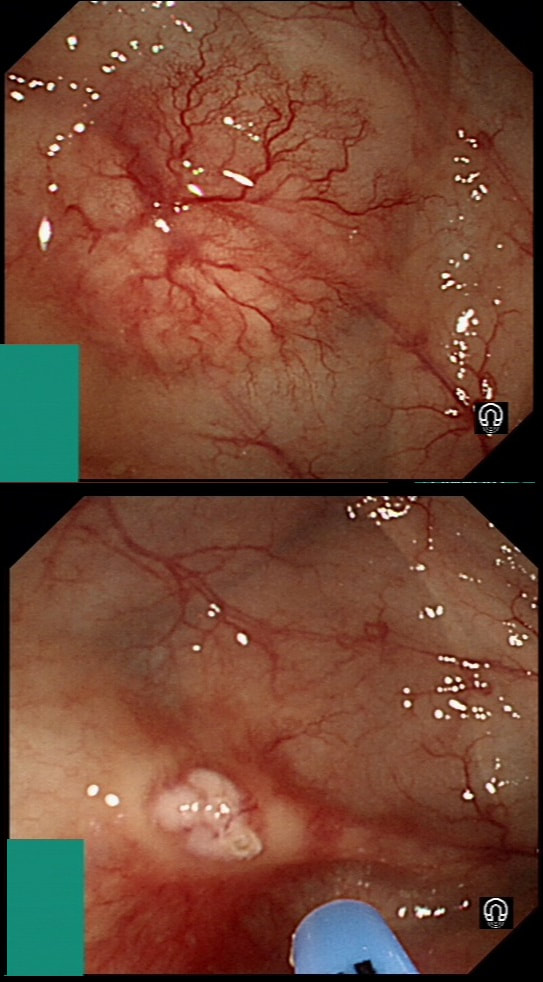
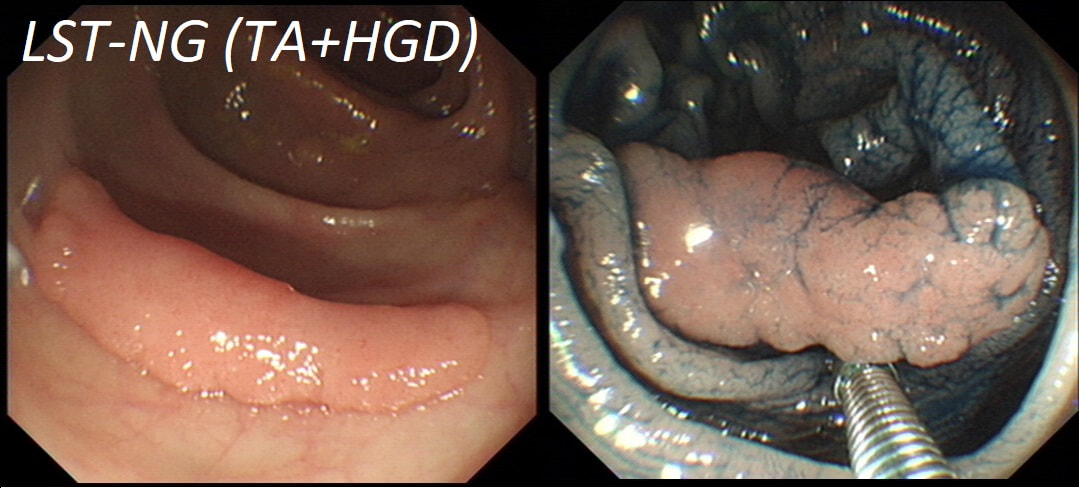
Here are three colonic polyps. One is a TA+LGD, another a TA+HGD and the final is a TVA+HGD
WHICH POLYP IS THE TVA?
a) A
Nope, this one has slit-like crypts typical of a tubular adenoma (TA). In fact, most small polyps are TA's. Presumably there is a progression from TA→TVA→Cancer apart from depressed lesions (IIc lesions) which go directly from being TA's to cancer.
b) B
It's a little difficult to see the crypt pattern without a close-up. However, you don't need to! Look at the growth morphology. This is a LST-NG and they are ALWAYS TA's!
c) C
Yes! Gyrate crypts best seen after indigocarmine dye spray (I think).
explanation
Most 'laterally spreading TVA's are of course LST-G's. However, this doesn't have the usual cobblestoned appearance of a 'laterally spreading tumour of the granular type'. It's rather 'chunky' in fact. That's because its harbouring HGD! A rare beast indeed as almost all laterally spreading TVA's only harbour LGD.
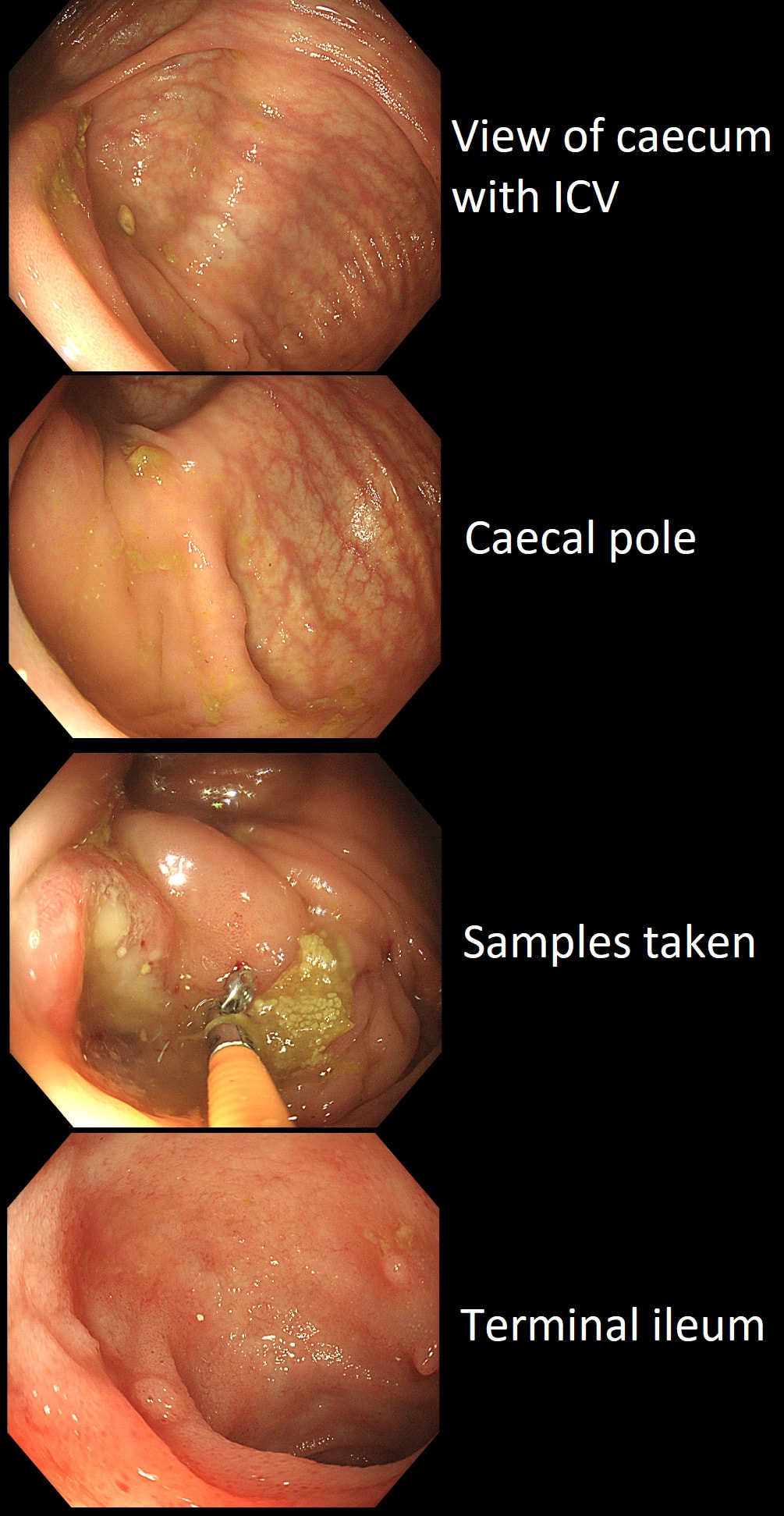
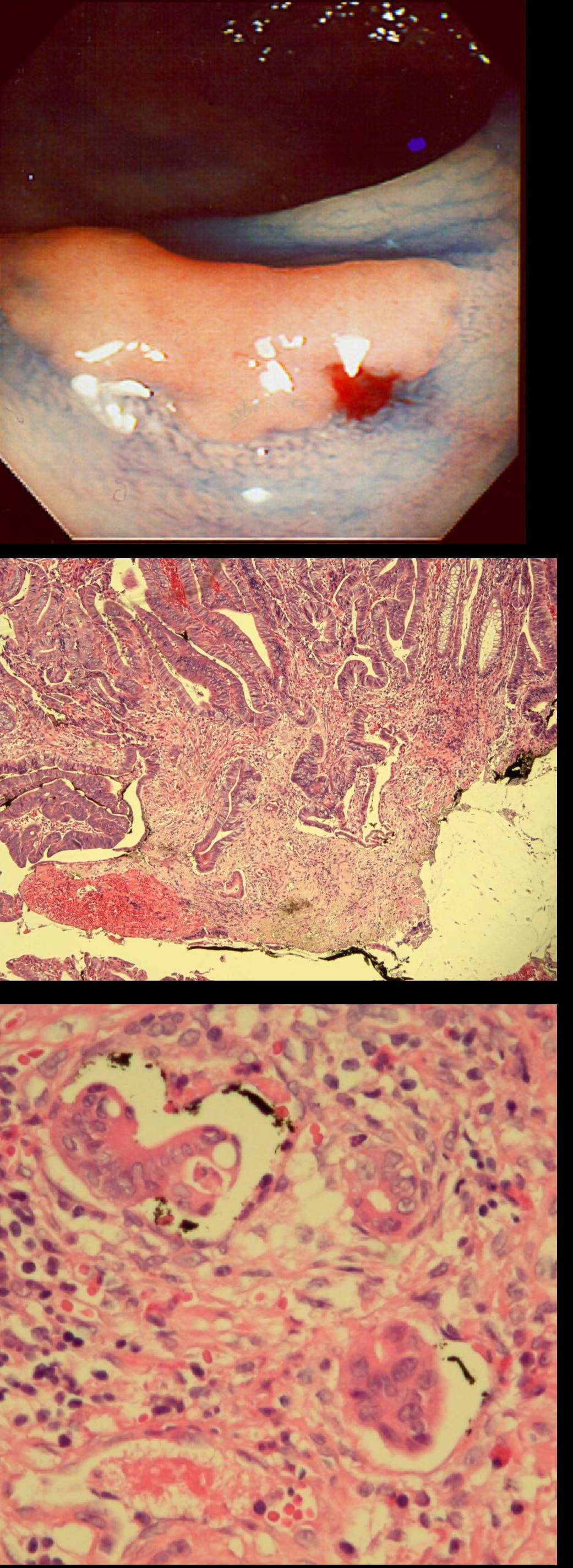
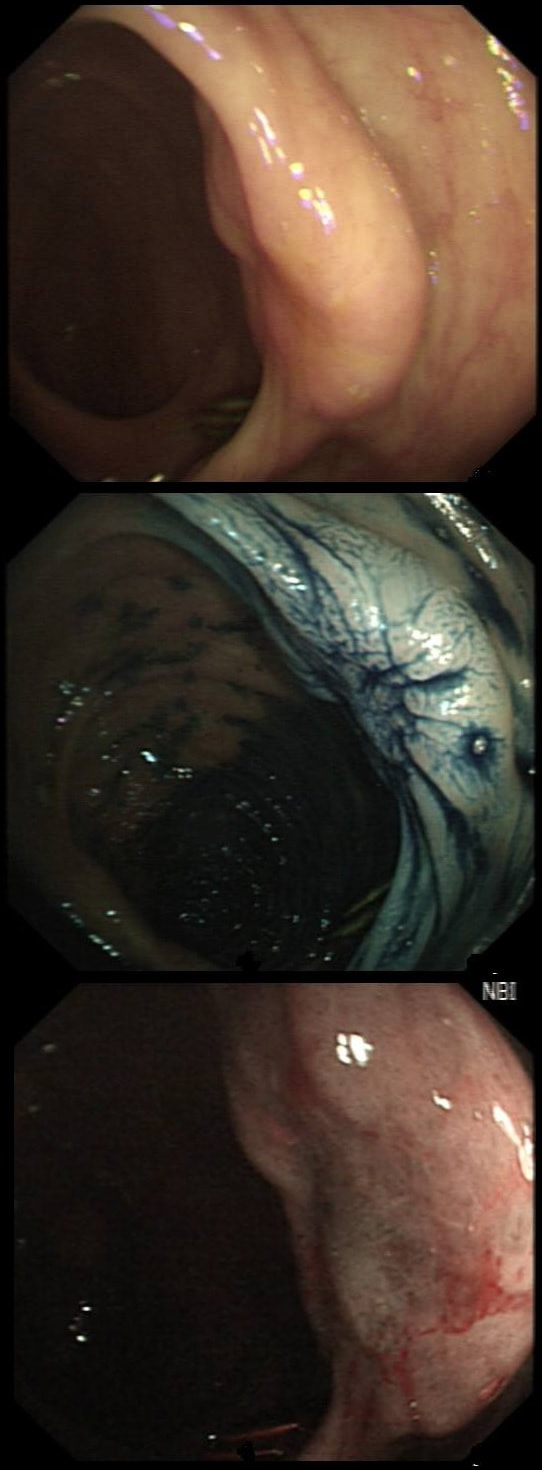
This patient underwent a CT angiogram for chest pain. The angiogram was unremarkable but the radiologist mentions an 'irregularity at the caecal pole with hyperenhancement and mild adjacent fat stranding' and recommend colonoscopy. After the examination, your patient asks you if you have found anything?
WHAT WILL YOU TELL YOUR PATIENT
a) Reassure the patient
That would be unwise!
b) Tell him to wait for the histology
Strictly speaking 'not wrong' but it's not the best option to choose
c) Tell him that we will organise an MRI scan next
MRI's are requested for rectal cancers
d) Tell him that we will organise an EUS next
Life is too short for EUS!
e) Tell him that we will organise a 'staging' chest/abdomen/pelvic CT
This is precisely what you should do!
explanation
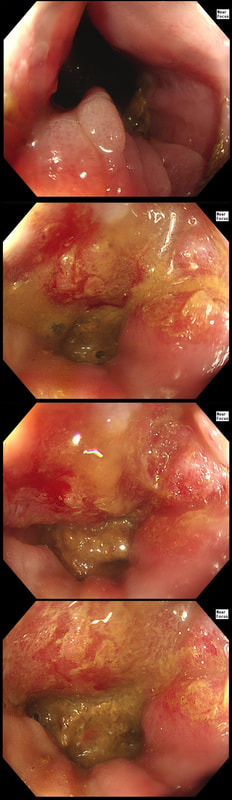
Did you notice the lesion next to the biopsy forceps? At initial glance into the caecum, there is nothing to see. However, this lesion was about 20mm in diameter and with that rolled edge. It's clearly malignant and you need to request staging CT's. Histology did confirm a mucinous adenocarcinoma and CT sized it at 4.7cm. Far larger than initially thought! As it was involving the serosal surface, it was staged as T4, N1 (due to several small nearby nodes).
Mucinous colonic cancers are unusual, accounting for about a little more than 10% of CRC's. They are usually situated in the proximal colon. This is not the only reason why they are easy to miss. At the early stages they have an infiltrative, ulcerative growth pattern which easily hides behind bubbles or a pool. These are small but evil little things which are easy to miss and grow fast. By the way, 'signet ring adenocarcinoma' (where the mucus is INSIDE the cell rather than OUTSIDE of the cells) is another sub-type of adenocarcinoma which may be part of the same spectrum. Mucinous and signet ring adenocarcinoma, share similar molecular features such as MSI-H, CpG island methylator phenotype-high (CIMP-H), and frequent BRAF V600E mutations. Of course microsatellite instability is linked with Lynch syndrome but in this case immunohistochemistry stains revealed normal mismatch repair proteins MLH1, PMS2, MSH2 and MSH6. This scary looking polyp was found in the sigmoid in a patient on a polyp surveillance programme. WHAT IS THE LIKELY DIAGNOSIS?
a) Mucosal prolapse
Yes! You can't see any typical gland openings at the tip of this polyp. It's a mucosal prolapse polyp with a serrated lesion at the tip.
b) Adenomatous polyp
It's a battered polyp but without any recognisable crypt openings at the tip and therefore NOT adenomatous.
c) Malignant polyp
Well it looks scary and without an organised crypt pattern. All hallmarks of a malignant polyp. However, this lacks crypts because it's an innocent mucosal prolapse polyp!
Explanation
You've seen a few examples now of the 'mucosal prolapsing conditions' which include solitary rectal ulcer syndrome and inflammatory cloacogenic polyps. First described in 1985 [GIE 1985;31:196–9] they are usually said to be rare. However, experienced endoscopists know that these are common in the sigmoid. I've never seen a mucosal prolapse polyp outside of the sigmoid and would actually not make this diagnosis elsewhere in the colon. Histology is usually said to be the way to "confirm the diagnosis and rule out cancer". However, for me it's an endoscopic diagnosis. But I do understand that these lesions do look alarming. For this reason, I sometimes take it upon myself to resect the tip of the lesion, proving to all those of little faith, that the lesion is absolutely innocent. However, I'm not sure that my surgical colleauges are always reassured by the histology which usually rambles on about 'distorted elongated branched crypts, with fibromuscular obliteration of lamina propria with lots of intramucosal haemorrhages and a splayed and hypertrophied muscularis mucosae". However, the bottom line of that pathology report will read; 'No dysplasia'.
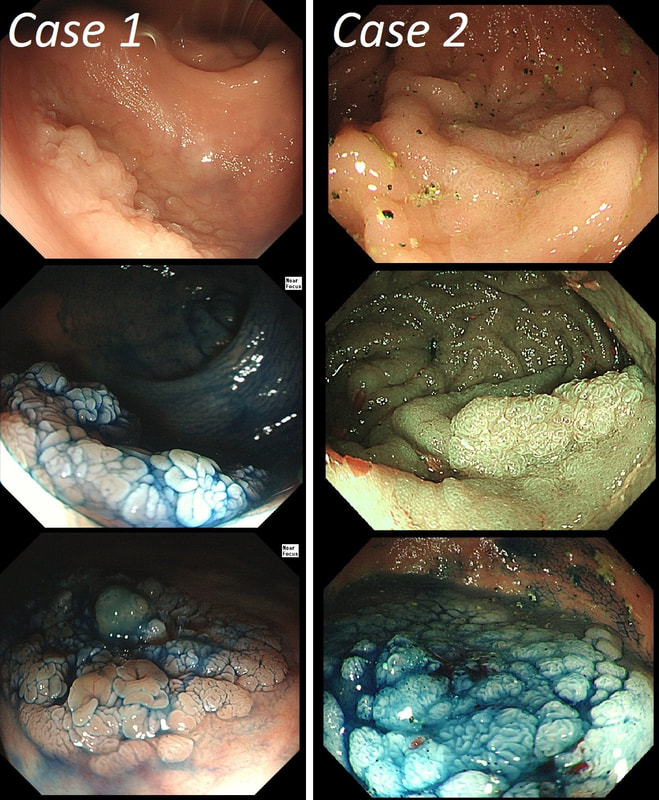
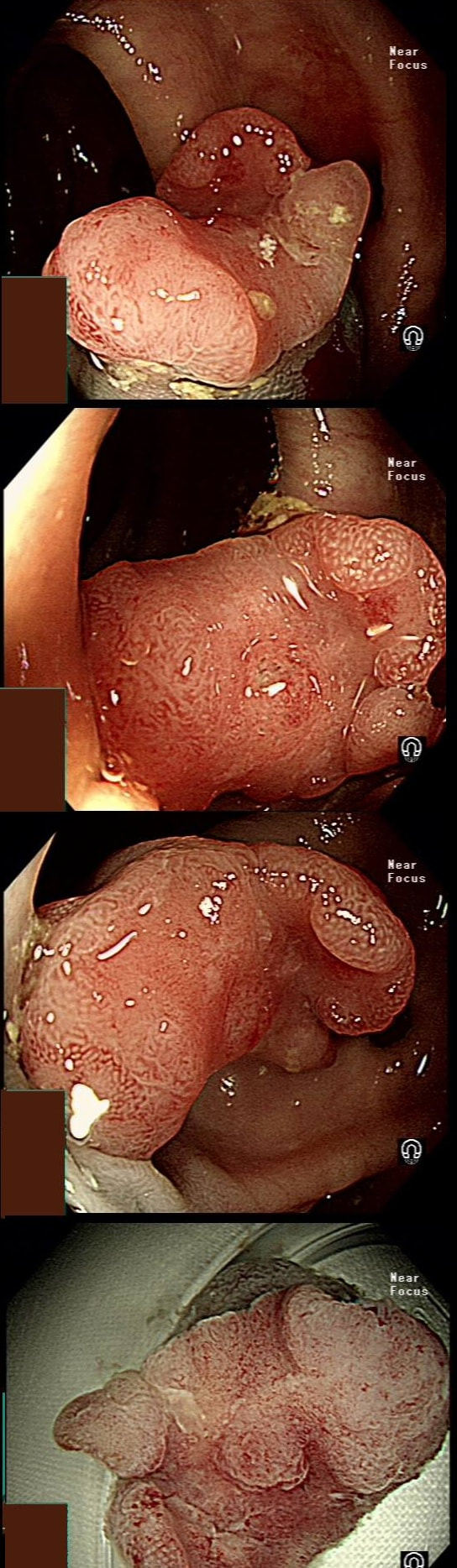
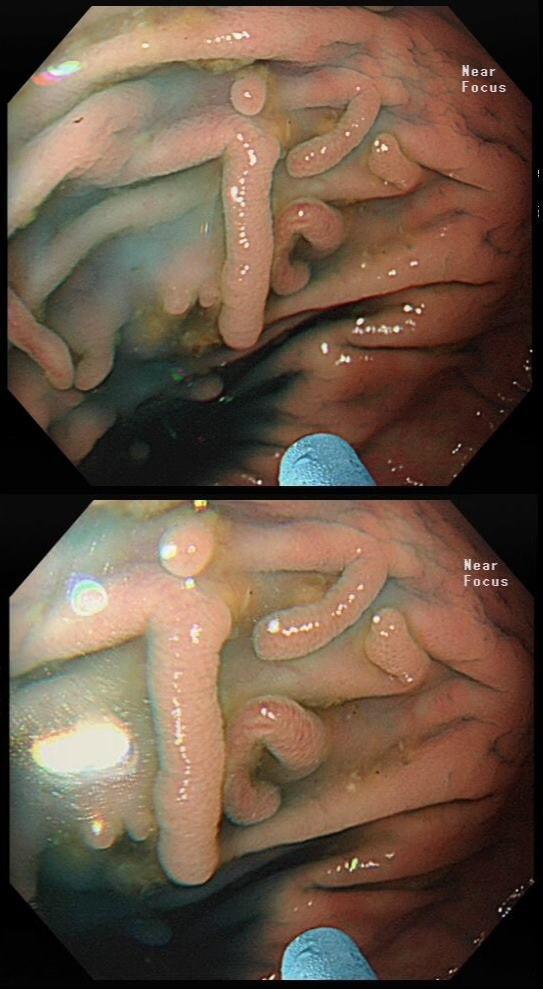
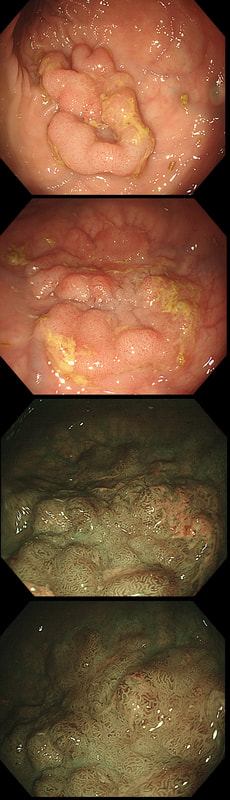
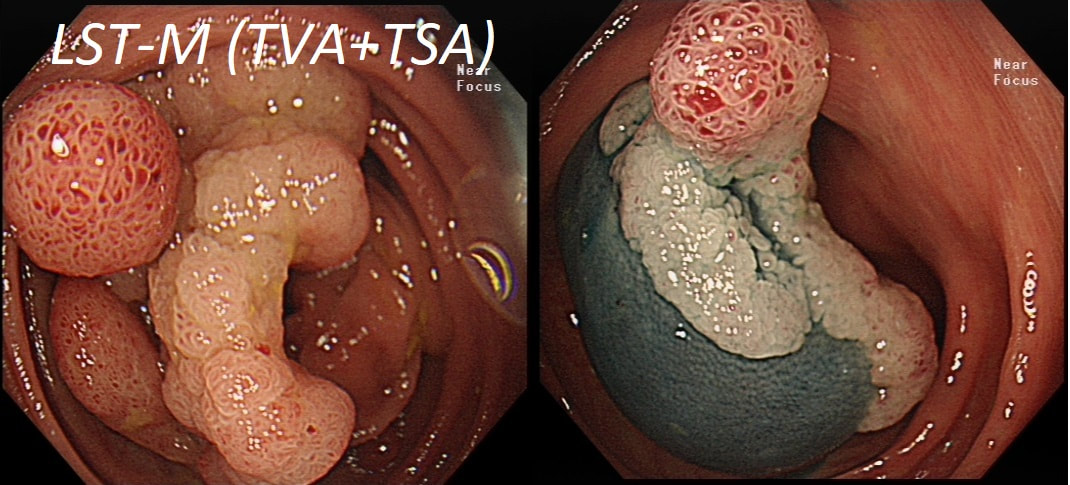
You may be surprised to hear that both these polyps (found in the caecum and rectum respectively) actually have the same histology!
WHAT IS THE LIKELY HISTOLOGY OF THESE TWO LESIONS?
a) SSL's
But it has gyrate crypts and perhaps villous in places??!
b) TSA's
Well done! TSA's are great cameleons !
c) TA's
Slit like or small round crypts? No !
d) TVA's
Reasonable guess as the growth morphology is that of a LST-G which are usually TVA's!
e) VA's
Right-hand image does look a little villous, perhaps
explanation
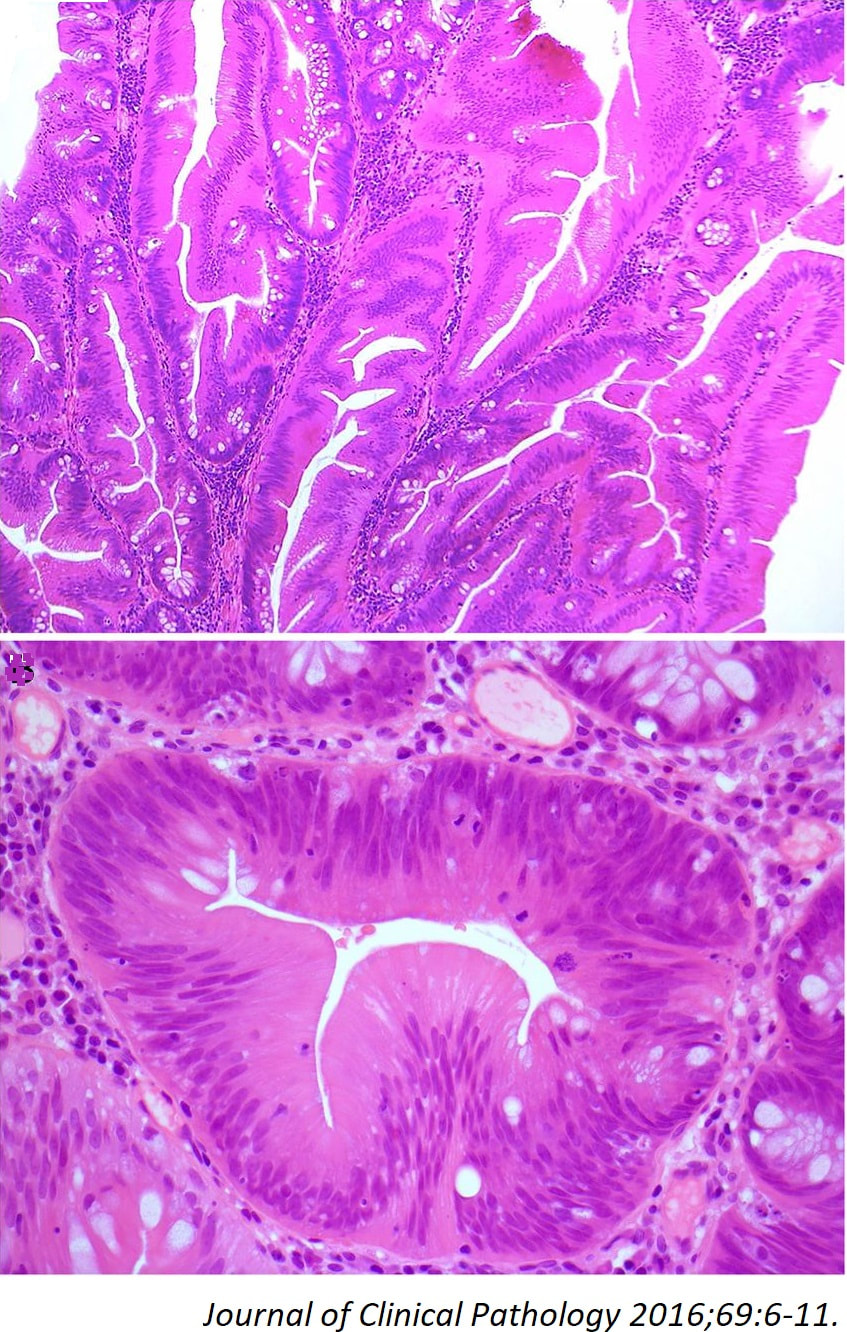
The surface appears villous in places and gyrate in other areas. The truth is that it is a bit of both - a 'Traditional Serrated Adenoma' (TSA), first described in 1990 by Longacre and Fenoglio-Preiser (Am J Surg Pathol 1990;14:524–37). TSA's are usually less recognisable and usually somewhat 'cerebriform' (like an exaggerated TVA).
TSAs are the least common of the three serrated colonic polyps ('hyperplastic polyps', 'sessile serrated lesions' and TSA’s) and account for only about 5% of serrated polyps and less than 1-2% of all colonic polyps and are usually found in the distal colon. Pathologists rely on three typical findings when diagnosing TSA’s: deeply eosinophilic cells, flat top luminal serrations and numerous ectopic crypt foci (all demonstrated in the histology slide below from the Journal of Clinical Pathology 2016;69:6-11). Genetically, TSA's are more like adenomatous polyps harbouring low-level microsatellite instability (MSI-L) or microsatellite stable (MSS) serrated colorectal adenocarcinomas. They usually contain KRAS mutations rather than usual BRAF mutations which you find in the 'Serrated Pathway' to CRC associated with a high-level of microsatellite instability (MSI-H). Nevertheless, it’s thought that these precursor lesions give rise to serrated colorectal adenocarcinomas as part of the serrated (accelerated) pathway to cancer.
This lesion was found in the ascending colon and referred for removal.
WHAT IS THE LIKELY DIAGNOSIS?
a) HP
You must be joking!
b) SSL
You are not serious?
c) TA
Short slit-like crypts, well Yes but there is more to it!
d) TVA
Absolutely not!
e) Cancer
That non-lifting sign doesn't lie!
explanation
Must admit that I didn't like the look of this polyp. Sure, it does have an organised crypt pattern. I think that I can see short slits, making it a TA. This would fit with the fact that it probably is a LST-NG type of lesion (which are always TA's).
However, it also looks, 'chunky' and it's the 'thickness' of the lesion which made me suspicious. For this reason I was not surprised to find the non-lifting sign. As there is no lifting what-so-ever, I suspect that the lesion will turn out to be T2 at least. This is an interesting looking colonic polyp WHAT IS THE LIKELY HISTOLOGY
a) mixed serrated / adenomatous polyp
And that is what it looks like!!!
b) traditional serrated adenoma
But there are TWO components to this polyp!
c) serrated polyp undergoing malignant conversion
Well something has happened but 'lesion' has beautiful crypts!
explanation
Actually, the red bit of the polyp was composed of a tubulovillous adenoma and the rest was a serrated polyp! In other words, a mixed serrated/adenomatous polyp. Clearly the famous "three pathways to colorectal cancer": the APC mutation pathway (50%-70%); the mutator “Lynch syndrome” route (3%-5%); and the serrated pathway (30%-35%) can sometime get mixed up !!! This lesion, situated in a very spastic sigmoid, has been referred for resection. WHAT IS THE LIKELY DIAGNOSIS?
a) lesion is not neoplastic
Yes, hard to believe but true!
b) lesion is adenomatous
Lesion is 'battered' but crypts don't look typically adenomatous
c) lesion is malignant
No way, lesion isn't angry red, chunky or devoid of crypts!
explanation
I couldn't stop myself! After yesterday's case of a rectal 'prolapse polyp, part of the 'Mucosal Prolapse Syndrome', I had to show an example of a sigmoid mucosal 'traction polyp' (my nomenclature). The mucosa at the apex of this sigmoid fold is traumatised and inflamed but not actually adenomatous! Histologically these lesions also appear somewhat bashed up. This is where pathologists may see 'pseudo-invasion' which is actually movement of crypts due to trauma and inflammation. The sigmoid colon is the most 'powerful' part of the colon developing the force needed to go to the toilet. Presumably this is the reason that diverticular disease first develop in the sigmoid. The force can also create these pseudo-polyps from patches of inflammation which I presume gets tugged along with each peristaltic wave. The end result is that this is the most difficult part of the colon to make head an tail of polyps. These are common lesions and if you are a 'therapeutic endoscopist', you will be refer these lesions. In these cases, I don't go overboard by placing a snare far down the 'pseudo-stalk'. If you did, you will find that it's taking a long time to cut through all that healthy sigmoid mucosal fold and you run the risk of a perforation (early or late). Instead, I just catch the tip of the fold and ask my assistant to close the snare as quickly as possible. Of course, you don't need to worry about a type of chunky central vessel which you may find in an adenomatous polyp. Analysis of a small piece of mucosal apex confirmed a normal mucosa. Hopefully this was enough for everyone to relax ... This lesion in the low rectum has been referred for endoscopic resection WHAT IS YOUR DIAGNOSIS?
a) Mucosal prolapse syndrome
Well done!
b) Sessile Serrated lesion
Looks just like it but it isn't!
c) Traditional Serrated Adenoma
That's a No!
d) Adenomatous polyp
Patient complained of constipation...
e) Malignant polyp
But it's not 'chunky', red and it has crypts ?!?
explanation
There is no distinct edge to the 'polyp' and the crypts look normal, although a little splayed out and larger than normal, reminiscent of a serrated polyp. In real life, I had the advantage of knowing that the patient had been sent for colonoscopy because of constipation. I low-risk indication of course. There is no distinct edge to the 'polyp' and the crypts look normal, just a little splayed out and larger than normal. This 'polyp' is actually part of the "Mucosal prolapse syndrome", first described in 1983 by Du Boulay et al [J Clin Pathol. 1983;36:1264–8]. Obviously, this syndrome includes solitary rectal ulcers, inflammatory cloacogenic polyps but also rectal 'prolapse polyps' such as this. In addition, the 'Mucosal Prolapse Syndrome' includes those prolapsing mucosal folds in the sigmoid and perhaps most surprisingly - GAVE ! In a case like the above, a couple of superficial biopsies will usually reveal the true nature of the lesion as it's easy for pathologists to spot the fibromuscular hyperplasia with overlying epithelial crypt distortion rather than dysplasia. This is a video clip of a small lesion removed from the sigmoid colon. WHAT IS THE MOST LIKELY HISTOLOGY?
a) TA+LGD
But it's a IIc lesion with IIIs crypts in the centre?!?
b) TA+HGD
Absolutely!
c) Invasive cancer
But there ARE small round crypts in the centre and it DOES lift!!!
explanation
You may call this a "flat elevated lesion with a central depression (IIa+IIc lesion) or simply a depressed lesion (IIc lesion). Frankly it doesn't matter because both a part of the same 'family' of evil little b.....ds. They are always TA's and the small, round crypts (Kudo type IIIs crypt pattern) tells you that the lesion harbours HGD. This is because as dysplasia progresses from low to high grade, crypts get smaller and more withered. Of course they eventually disappear altogether as the lesion develops into a cancer which no longer follows any 'instructions' to form organised crypts. However, the crypt pattern is still discernible in the centre AND the lesion lifts well. Both of these tells you that the lesion is likely to still be benign. Ultimately, the pathologists called it a TA+HGD. However, there was mucinous differentiation in the centre of the lesion. Could these little shits be the early stage of mucinous colonic cancers? Quite likely! Imagine how easily they are missed when hiding behind a fold or below a shallow puddle !!!
A polyp found in the descending colon and removed as a single fragment (H&E attached)
WHAT IS THE DIAGNOSIS
a) Tubular adenoma
Yes but you are only partly correct!
b) Tubulovillous adenoma
No way, this is a LST-NG!!!
c) Villous adenoma
Absolutely not - there are no villi!!!
d) Sessile serrated lesion
Looks like it but histology doesn't!
e) Malignant polyp
Full marks!
explanation
This is a LST-NG type of lesion (laterally spreading tumour of the non-granular type). They are always TA's (tubular adenomas) and often (but usually not), harbour HGD or cancer. I guess that we can't really be sure about the crypt pattern as this is a non-magnified image. However, looking at the histology slide with narrow crypts, I expect that the crypt pattern is probably IIIs (small round crypts) which goes with TA+HGD. Must admit that I was surprised to find invasive cancer and LVI (lymphovascular invasion) in a small lesion such as this! The last image shows clusters of malignant cells within lymphatics. Of all the 'markers' to suggest that the patient needs surgery, LVI is the most important!
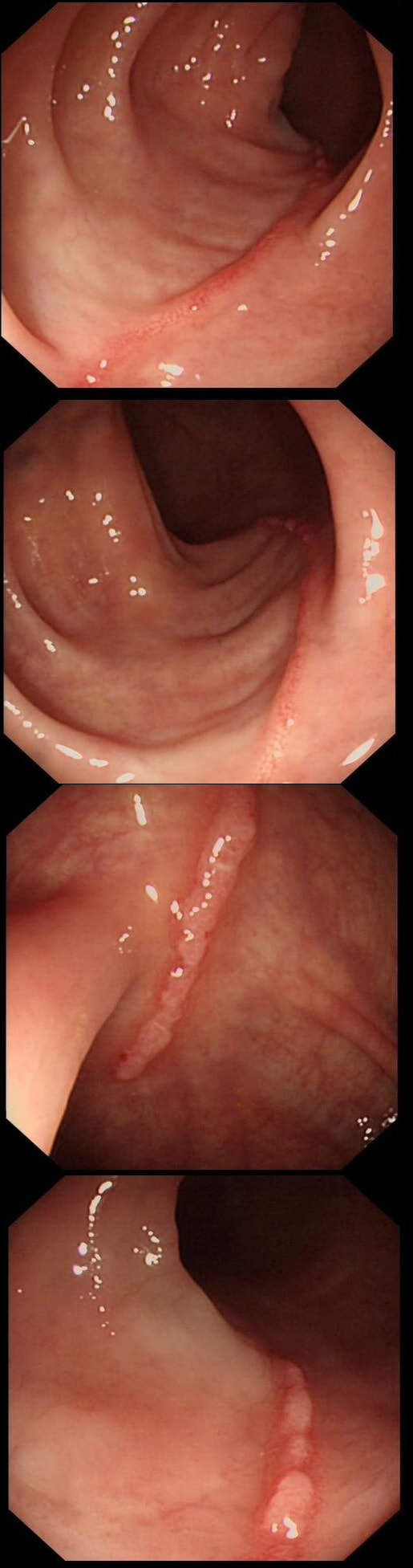
These linear lesions were found in a 50 yr old patient undergoing colonoscopy to investigate her loose stool.
WHAT IS THE LIKELY DIAGNOSIS?
a) Crohn's disease
Does give linear ulcers but should be larger and deeper!
b) Ischaemic colitis
Also gives linear ulceration, often on the ante-mesenteric border.
c) Collagenous colitis
Absolutely!
d) Ulcerative colitis
Doesn't give linear ulceration and surrounding mucosa wouldn't be normal
e) Lymphocytic colitis
Perhaps one could develop into the other?
explanation
Actually, this patient had collagenous colitis! I suspect that the acute injury are linear tears in the mucosa which then granulate as in the images above. Finally, you are left with linear scars as in the image below. Pure speculation but seems logical ! At colonoscopy, the mucosa is often unremarkable but there may also be mild, patchy erythema or linear cracks. Histology reveals the typical thickening of the subepithelial collagen layer from the normal 3-6 μm to more than 10 μm as well as lymphocytic infiltration of the epithelium and the lamina propria. Rectal biopsies are not sufficient to make the diagnosis as normally the collagen layer is particularly thin here. Samples from the rectum and sigmoid confirms the diagnosis is more than 90% of cases. Interestingly, patients with eosinophilic oesophagitis may also develop some fibrosis in the lamina propria which also 'cracks' in a spectacular way when a dilatation is carried out.
This polyp is pretty when viewed by the endoscopy but stunning below the microscope!
WHAT IS THE HISTOLOGY?
a) Tubular adenoma (TA)
This polyp is prettier than that!
b) Tubulovillous adenoma (TVA)
Endoscopically it could be but histology says otherwise!
c) Villous adenoma (VA)
Absolutely!
d) Traditional Serrated Adenoma (TSA)
Endoscopically Yes, but histologically No!
e) Sessile Serrated Lesion (SSL)
Would be unusal for an SSL to be 'subpedunculated'
explanation
Endoscopically it can be difficult to recognise a villous adenoma. Unless you submerge the lesion to see the villi rise up like the arms of a sea anemone. Apparently, they are quite difficult to 'process' by the pathology lab as well. They are fragile and those beautiful villi, easily become damaged.
Of course, villous adenomas are regarded as 'high risk lesions'. The other high risk findings often used as surrogate markers for cancer in guidelines are: having 3 or more adenomas, polyps 10mm or larger, and polyps harbouring high-grade dysplasia. However, if you have to make a choice of the two strongest predictors of future cancer risk, a study in Gastroenterology found that it would be; finding a polyp 2cm or larger and resecting a polyp found to harbour HGD [Gastroenterology 2019;158(4);875-83].
This colonic polyp was removed as a single fragment from a 60 year old lady. You can see the mucosal defect in the last image. The patient asks you what will happen next?
WHAT WILL YOU REPLY?
a) Can't tell, we will have to wait for the histology
You can do better!
b) In all likelihood there will be a site-check in 4 month or so
You are missing something!
d) You will probably need an operation
You've spotted the desmoplastic reaction!
explanation
The polyp looks very suspicious but did seem to lift and I therefore decided to go ahead and removed it using a stiff, large snare. It took a little longer than expected for the snare to cut through. Of course, the mucosal defect should be blue. In this case it's yellow! The polyp was malignant, invading about 1mm into the submucosa and you are looking at the 'desmoplastic' reaction generated by the cancer.
Apart from the sometimes deceptive 'non-lifting sign', there are two further signs that a lesion may be malignant. First, it may look smaller and smaller as you inject below the lesion (see example below). Another sign is that your blue sub-mucosal injection appears to lift the lesion until you retrovert and have a look at the other side. If you then find that it hasn't actually 'crossed the mid-line', there is fibrosis below the lesion preventing the fluid to disperse evenly. I was not entirely surprised to learn that the patient declined surgery. After all, he was 86 years old! He lived another 7 years and never developed any sign of bowel cancer. By the way, there is a theoretical risk of tumour seeding if the lesion is perforated during resection. However, when the perforation is done with a red-hot tool such as a knife or snare, the risk of seeding is surprisingly low. I have perforated a handful of cancers but have never had a case of late disseminated peritoneal disease. My Japanese colleagues (off the record), agree that the risk is there (some have seen it) but is low. If you decide to sample a suspicious looking polyp, you shouldn't use the same forceps to sample another lesion. This is because if cancer cells become lodged in the biopsy forceps, which are then used to sample another location and them become stuck in the biopsy, the histopathologist will diagnose cancer in TWO locations when in fact, there is only a single cancer.
This patient with ulcerative colitis has developed a polyp in the transverse colon. The lesion has now been sent for an endoscopic resection.
WHAT WOULD YOU DO?
a) Abort!
Smart!
b) Attack!
You are creating a problem of your own making!
explanation
Some would say; "if you can remove the lesion in that colitic colon, then 'do it'! The problem is that nothing may appear "irresectable" giving plenty of time, determination and poor judgement.
Many studies looking at outcomes of polypectomy in UC, excluded polyps >1-2cm or flat polyps. Other studies have included polyps arising outside of the colitic field or only have a short follow up period of a few years. Actually, most are coming to believe that when dysplasia develops in the colitic colon, it's not a 'random' case of bad luck. It can be the result of a long process of progressive DNA damage! At some stage we will be able to have a look at the state of the stem cell DNA in patients with conditions such as Barrett's, Colitis and atrophic gastritis. I think that we are in for a surprise ! In addition, did you spot the small focus of invasive cancer in the 2-3 O'clock position? Surprisingly, this was only T1 disease!
A polyp found in the transverse colon
WHAT IS YOUR ENDOSCOPIC DIAGNOSIS?
a) Tubular adenoma
You are half correct!
b) Tubulovillous adenoma
On the left side, the crypt's are slit-like rather than gyrate
c) Villous adenoma
VA's are soft like sea anenome !
d) Serrated polyp
No, the crypts are not large, round openings!
e) Malignant polyp
Yes, there are no crypts on the right-hand side !
explanation
Did you spot that cancer has developed on the right side of this tubular adenoma (TA). On the left side, the crypts are slit-like whilst on the right hand side, the crypt pattern has been destroyed (Kudo type V crypt pattern). The Haggitt stage was I and I took particular care in removing the polyp with a large margin. The cancer was only 4mm in diameter. Perhaps the smallest adenocarcinoma of the colon you will ever see ?
This is the scar following the piecemeal removal of a sigmoid polyp some 6 months ago. It was a 15mm tubular adenoma harbouring high-grade dysplasia. Samples from the EMR scar has showed 'distorted glands' only.
WHAT WOULD BE THE CORRECT FOLLOW UP?
a) organise an immediate follow-up
WTF ! This not a normal scar! You absolutely need to organise more samples, and perhaps a CT !!!
b) organise a follow-up in 6 months time
Would have been a mistake !
c) organise a follow-up in 12 months time
Would have been a big mistake!!!
d) organise a follow-up in 3 years time
May have cost the patient his life!!!
explanation
The first EMR was piecemeal and histology could of course not confirm that the resection had been complete. Indeed the 'index histology' reads oddly mentioning "frequent mitotic figures" and "back to back glands". To a gastroenterologist these words does not sound particularly alarming.
However, the pathologist was trying to say "this looks like cancer but I can't actually make that diagnosis" !!! Indeed this doesn't look like a normal EMR scar! The whole area is indurated as if there is an infiltrative process below the mucosa. Histology was reassuring mentioning some distorted crypts only. Sadly, the endoscopist was content with the reassuring repeat histology and did not reflect on the worrying endoscopic appearances. He did NOT organise a second round of post-EMR samples and the patient returned 2 years later with an advanced cancer. The take home messages from this sad story?
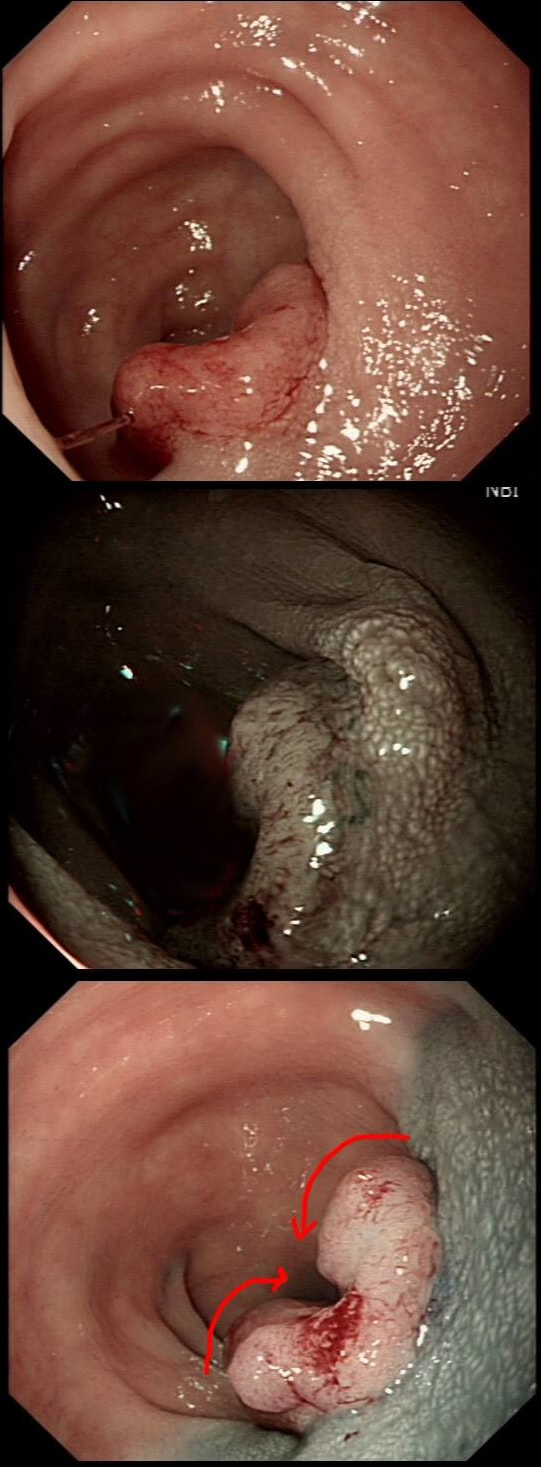
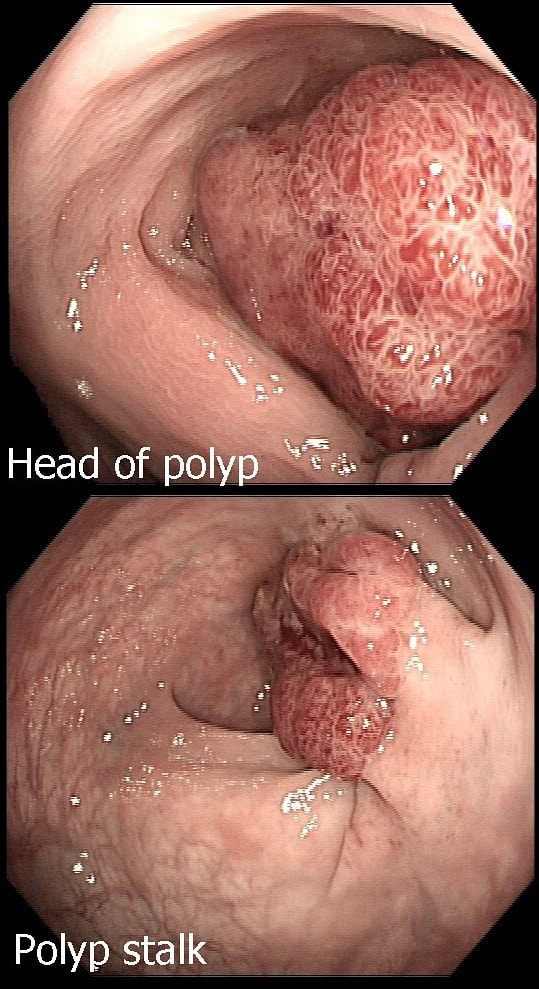
This polyp on a short stalk was removed from the colon
WHAT IS THE LIKELY HISTOLOGY?
a) TA
At the edge, the crypts are clearly sli-like but how about the centre?
b) TVA
Doesn't look like gyrate crypts!
c) VA
Doesn't look like a sea anenome!
d) TSA
TSA's do have crypts but this lesion doesn't in the centre!
e) Early cancer
Was my own firm diagnosis!
explanation
The head of the lesion is clearly of concern. There are 'horns' on it! Of course, the devil has horns but as it was arising from a stumpy stalk. I went ahead and removed it. Somewhat surprising our pathologists called the lesion TA+HGD!
Admittedly, there was disagreement between our histopathologists and 2 out of 5 believed that it was an invasive cancer. Endoscopically, the lesion is clearly malignant and at the very least it's an intramucosal cancer (IMca). Of course, our UK histopathologists are unable to make the diagnosis of Intramucosal cancer in the colorectum because this is not a diagnosis recognised by the 'Vienna classification'. Elsewhere in the GI tract, intramucosal cancer is a diagnosis which our pathologists are 'free' to make. It makes no sense whatsoever to me !
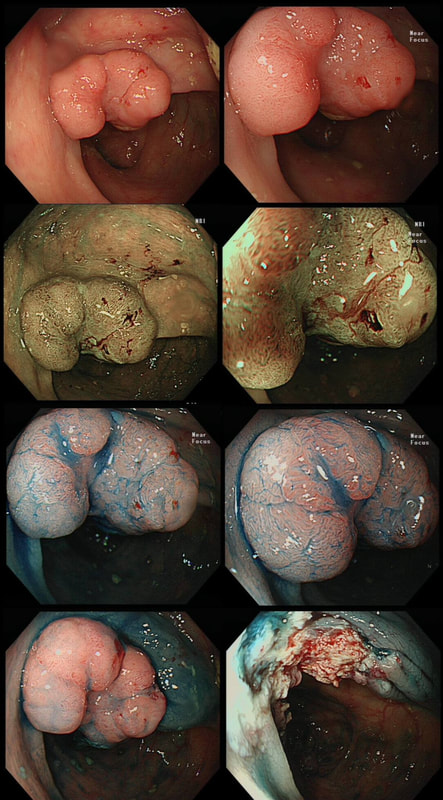
This is an odd looking sigmoid polyp which is seen arising from a broad stalk.
WHAT IS YOUR ENDOSCOPIC DIAGNOSIS?
■ Tubular adenoma (TA)
But the crypts don't look slit-like do they?
■ Tubulovillous adenoma (TVA)
Was my first thought but it doesn't look quite right...
■ Villous adenoma (VA)
If your bx forceps disappears into the polyp it's a VA
■ Traditional Serrated adenoma (TSA)
You'd be excused if you got this wrong - these are rare beasts!
explanation
My initial endoscopic diagnosis was that of a TVA. However, it looked a little "exaggerated". Actually it turned out to be a "traditional serrated adenoma" (TSA).
TSA's are rare and mysterious polyps. They only accounting for about 1% of colorectal polyps and are most common in the sigmoid and rectum. Endoscopically they can appear as “exaggerated” tubulo-villous adenomas or as villous adenomas. In the image below, I've put four examples of TSA's together to illustrate how differently these can appear ! It has been proposed that because one-third of "serrated cancers" are found in the distal colon and perhaps these cancers arise from TSA’s! However, genetically, TSA's are more like adenomatous polyps harbouring low-level microsatellite instability (MSI-L). They usually contain KRAS mutations rather than the BRAF mutations which you find in the 'Sessile Serrated Lesions'. For this reason, it's perhaps unlikely that they contribute to the 'Serrated Pathway' to cancer. By the way, a recent paper has highlighted that patients with TSA's are also at greater risk of 'high risk polyps', elsewhere in the colon.
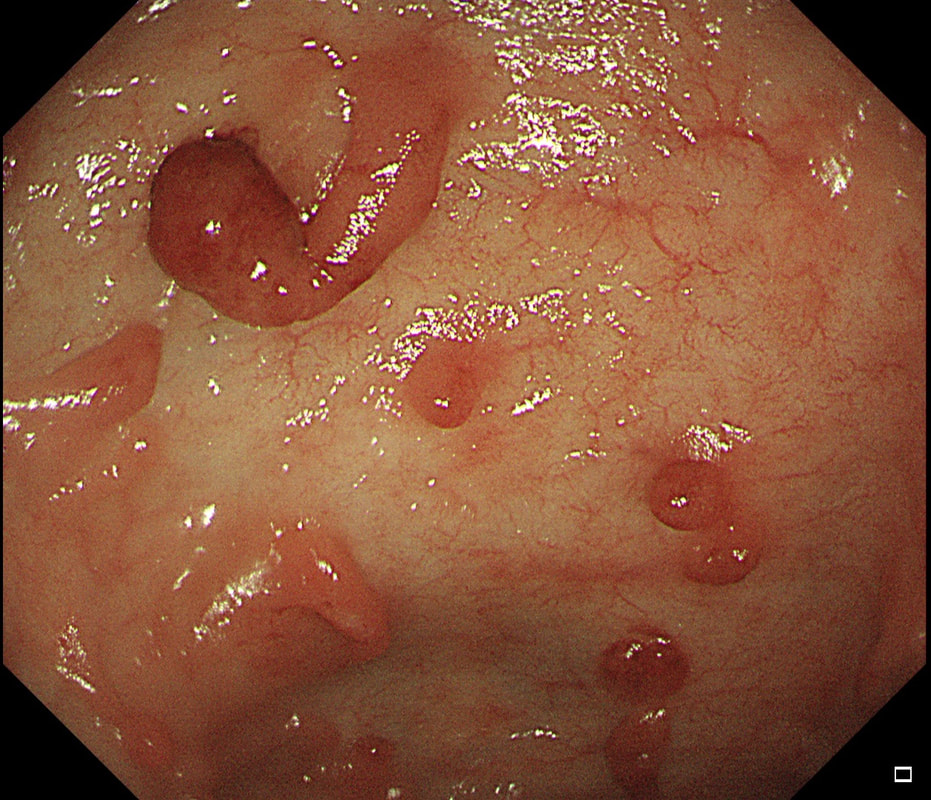
These peculiar colonic lesions were found in the colon of an asymptomatic patient.
WHAT ARE THEY?
■ Inflammatory polyps
Absolutely!
■ Adenomatous polyps
These are too long and peculiar looking to be adenomatous!
■ Hamartomatous polyps
No way!
This lesion was found in the transverse colon of a patient with iron deficiency anaemia (IDA).
HOW WILL YOU APPROACH IT WITH APC?
■ Zap the centre first
Absolutely, ablate the central feeding vessel!
■ Start in periphery and work towards centre
Would work but take longer than neccessary
■ In a spoke an wheel pattern starting in the periphery
Would also work but would take longer
This patient was referred for a flexible sigmoidoscopy because of PR bleeding. However, the only abnormality found was a sore anal canal. Samples are taken of course.
HOW WOULD YOU NOW ADVICE THE PATIENT?
■ We'll see you in clinic once histology is to hand
But there is a degree of urgency here!
■ Symptoms may improve with 'anusol'
No they will not!
■ We will try topical mononitrate first
You are barking up the wrong tree!
■ We will organise a scan next
Yes, a rectal MRI revealed something important!
■ Inject 80mg of triamcolonone
You are missing the point!
explanation
Actually, this isn't a case of haemorrhoids or an anal fissure. Histology reported; " Within hyperkeratotic epidermis there are scattered individual highly atypical infiltrating malignant cells with frequent apoptosis and moderate clear cytoplasm. There is no ulceration or significant inflammation."
Actually, this is a case of Perianal Paget's disease, - a VERY rare condition!!! You'll remember that a 'puckering' of the skin around the breast areola is associated with underlying breast cancer. This was first reported by Sir James Paget in 1874. However, a few years later, the same phenomenon was described elsewhere, so called "extramammary Paget's disease". In descending order of frequency, this has been described at; the skin of the vulva, perineal skin, perianal skin and the skin of the scrotum. Paget's disease, is usually NOT a primary cancer of the apocrine glands of the skin. It's almost always secondary to a nearby cancer of the rectum, anus or prostate. In this particular case, further imaging revealed a nearby prostate cancer! !
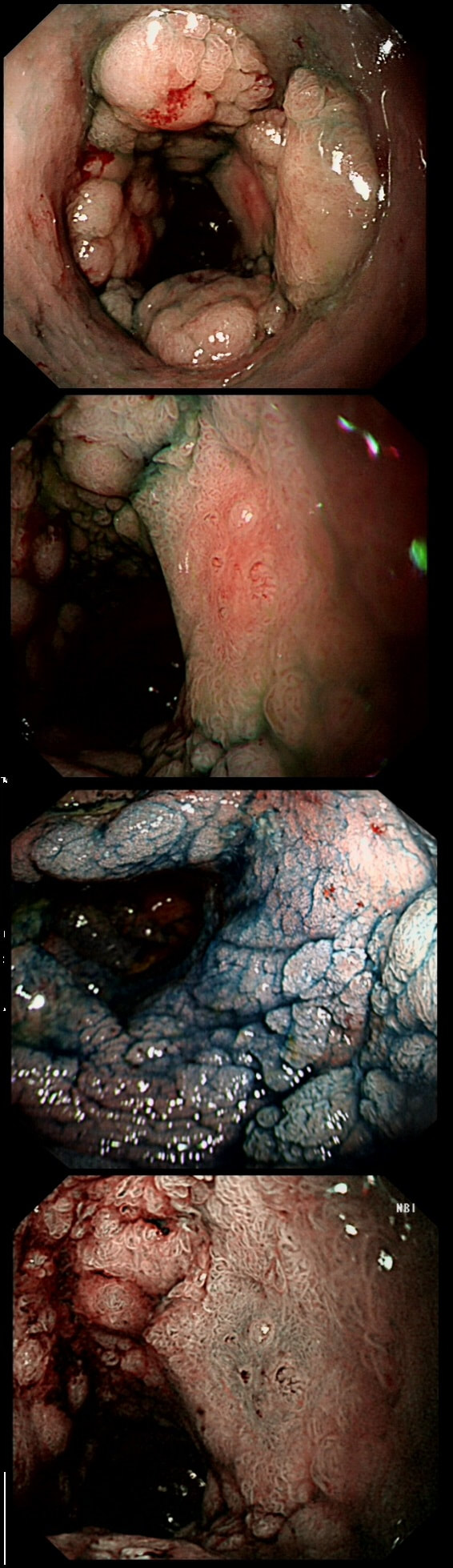
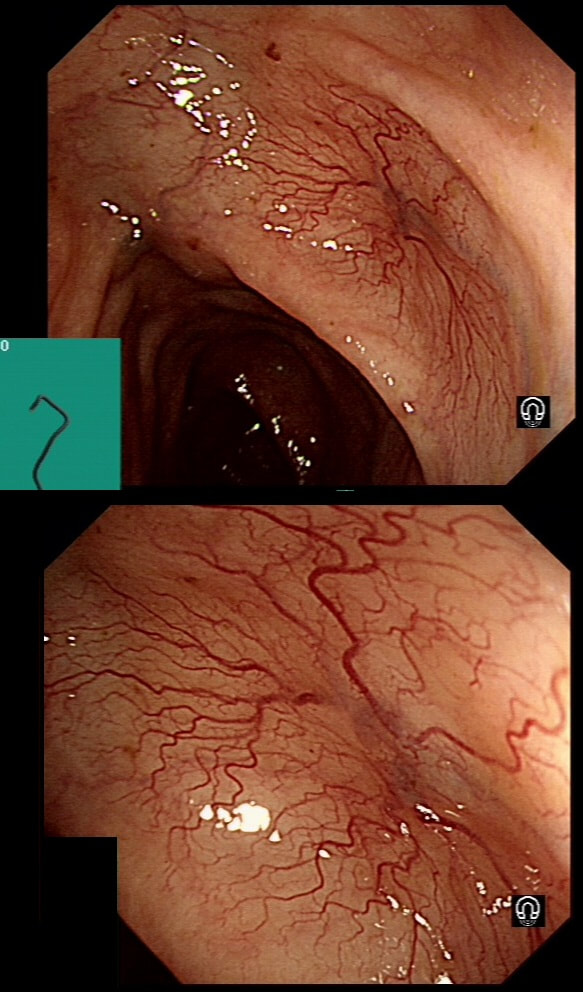
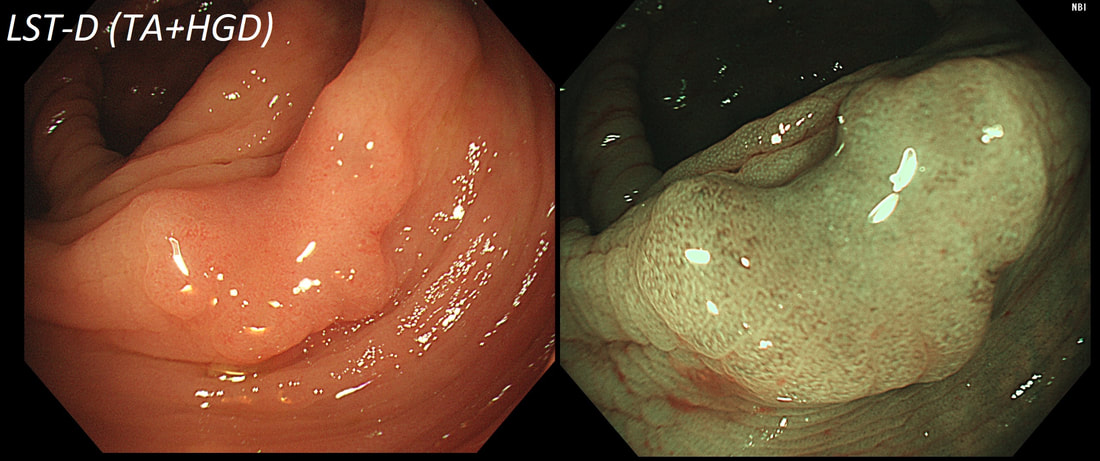
This lesion was found in the ascending colon.
HOW WOULD YOU DESCRIBE IT?
■ Flat elevated lesion (IIa)
Only lesions 10mm or smaller are referred to as 'IIa lesions'
■ Flat elevated lesion with a central depression (IIa+IIc)
But that depression dissappear when lumen is inflated!
■ Laterally spreading tumour of the non-granular type (LST-NG)
But the surface is 'cobbled' not smooth!
■ Laterally spreading tumour with a central depression (LST-D)
LST-D's are always TA's (and therefore have a smooth surface)
explanation
Lets just state something from the start! A flat elevated polyp is of course a IIa type of lesion BUT when larger than 10mm in diameter is no longer referred to as a IIa lesion. Now, it's a 'Laterally Spreading Tumour'!
There are 4 types as follows:
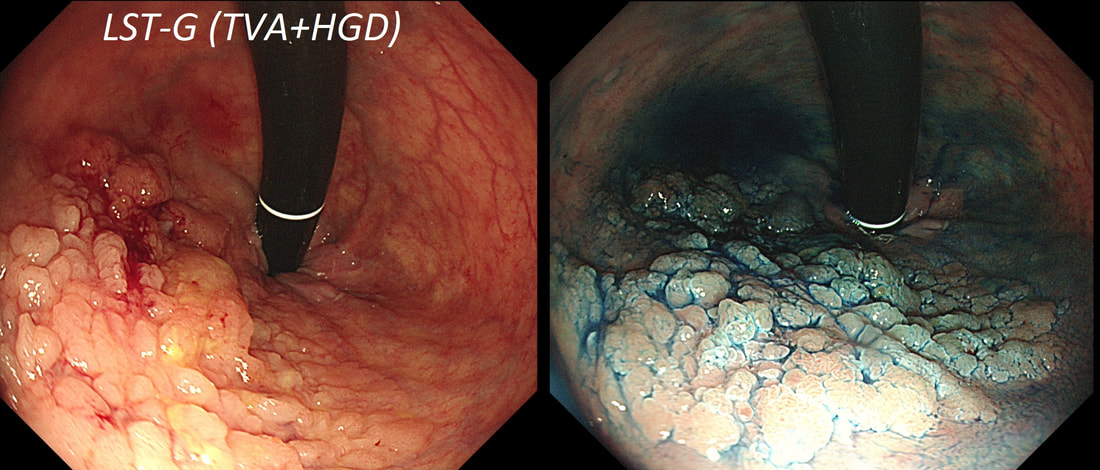
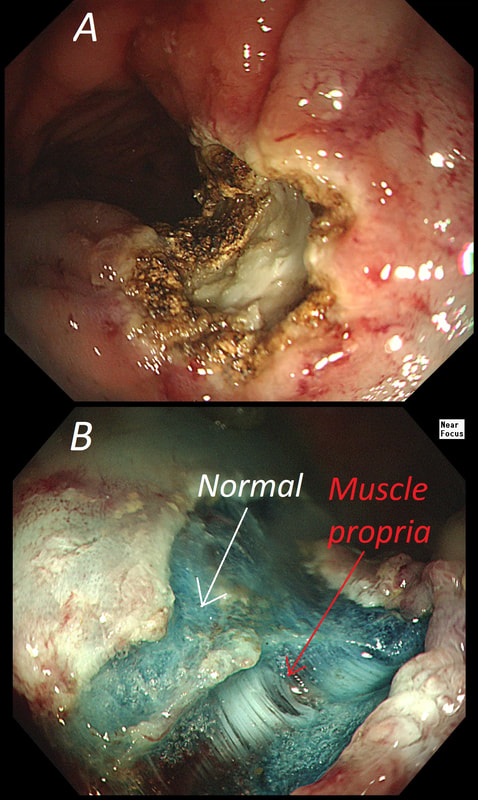
In this case the crypts are gyrate, (type IV crypts), making it a TVA. Laterally Spreading Tumours with a gyrate crypt pattern are all TVA's and almost never harbouring more than LGD. OK but it looks depressed in the centre doesn't it? No, it isn't depressed in the centre! It's simply they way the large lesion has folded itself. This is something of a 'trick question' as I deliberately didn't inflate the colonic lumen fully in the first image. When the lumen is inflated, you can see the true shape of the lesion; a LST-G ! A meta-analysis in 2013 by Voorham et al. [Voorham QJM. AM J Gastro 2013;108(7):1042-] concluded that pedunculated polyps were more likely to harbour KRAS mutation and APC mutations than flat lesions, and that flat lesions were more likely to harbour BRAF mutations. Depressed lesions and LST-NG’s were particularly unlikely to contain KRAS. Below are some examples of LST's Previous biopsies have confirmed that this rectal polyp harbours TVA+HGD. It's removed by piecemeal EMR and at the end of the 2min video clip you see the end result. WHAT WOULD YOU DO NEXT?
■ Place clips
Always clips !
■ Wait for histology
And of course, you have requested an 'urgent report'!
■ Request EUS
Life is too short for EUS!
■ Organise an MRI
For local staging and confirmed T2,N1 cancer
■ Request staging CT
Looking for mets in the Chest+Abdomen+Pelvis - proved negative!
explanation
The learning point of this video clip is the EMR defect; it's white (image A) !!! Perhaps the picture below explains it better. Normally my EMR defects are blue (because I mix indigo carmine dye into the submucosal mix). In 'image B' below, the white arrow in the second image shows you what a 'healthy EMR defect should look like. IF you cut too deep, you can see the white, linear fibres of the muscle propria layer (red arrow). Of course this is a warning sign that you MUST carefully close the defect with lots of clips. Actually, next to the tip of the red arrow you can see a black 'micro-perforation' where the full thickness of the muscle layer has been breeched. Naturally, this is were your first clips goes!
Anyway, the mucosal defect in the video clip is just - white, without any linear muscle fibres. This is fibrosis! I have seen fibrosis like this below large sigmoid polyps which have been yanked about with the forceful sigmoid peristalsis. However, the more usual reason for this appearance is that you are looking at the fibrous tissue below a cancer, called 'desmoplasia'. Consequently, if I see a fibrous tissue in the base of the lesion I would do the following: Place clips (because I always do) Fast-track the histology Organise an MRI Request staging CT (chest+abdomen+pelvis) The ultimate diagnosis? Histology confirmed that the polyp was malignant and the imaging (of course requested at the time of the resection), confirmed a T2, N1 carcinoma. A week later, we had a full diagnosis. Unfortunately, the patient turns out not to be a surgical candidate. There is rarely unbridled joy after the endoscopic removal of a CRC ... This sizeable polyp was discovered at the ileo-caecal valve and was referred for resection. Samples have not been taken to avoid tethering down making the endoscopic resection uneccessarily difficult. HOW WOULD YOU APPROACH THE LESION?
■ Back off and take some samples
Yes, and perhaps request a CT!
■ Attempt an EMR
You first need to decide what this is!
■ Consider removal by ESD
Can't be adenomatous, it's growing out of the TI!
■ Underwater EMR
Would also be ill advised
■ Refer for surgery
If this is what you think it is, then Yes!
explanation
In most cases, I would say 'if it lifts it will shift'. However, in this case I wouldn't bother with a test-lift. The reason is that the thing is growing out of the terminal ileum. You don't get adenomatous polyps growing out of the TI! This must be something else! In fact the true lesion may be larger than the red nipple-like polyp. Even though there are no large 'tell-tale' vessels running up it's side, the only thing of this size, growing out of the TI, is a NET! A lymphoma was my second guess. TI NET's are often bad news and should be considered for surgical resection. There is another odd thing about NET's growing in the terminal ileum. The WHO grading system doesn't seem to relate to the aggressiveness of the lesions behaviour ! This was only WHO grade I (proliferation index was only 1.8%) but on a full analysis after the right hemi-colectomy, the NET was found to be a invading into the muscle propria layer and with metastatic deposits in 2 out of 12 resected lymph nodes (T2,N1) as well as ulcerated deposits of NET in the nearby pericolic fat on the serosal surface. The moral of the story? In the terminal ileum, 'well differentiated NET' doesn't mean that it's well behaved!
This lesion was found in the rectum of a patient undergoing colonoscopy because of constipation
WHAT IS THE LIKELY DIAGNOSIS?
■ Polyp from mucosal prolapse
Clever!
■ Serrated polyp
Crypts look a little serrated but the rest doesn't
■ Adenomatous polyp
Polyp doesn't look 'neoplastic'
■ Malignant polyp
Now, this doesn't look malignant!
explanation
Well perhaps the crypts look a little like serrated crypt openings but somehow the rest of the polyp doesn't look like a typical serrated lesion. Where is the covering mucus?! Furthermore, the polyp definitely doesn't look adenomatous or malignant!
Actually this has arisen as part of the 'Mucosal prolapse syndrome' which is the umbrella term for entities such as; solitary rectal ulcer syndrome and inflammatory cloacogenic polyps. Patients are often constipated or have difficulty with defaecation with straining on the toilet or undergo the sigmoidoscopy because of tenesmus, altered bowel habits or incontinence. Surprisingly, some patients don't have any straining-related complaints! Most pathologists would recognise the typical mild fibrosis, thickening of the muscularis mucosae and crypt irregularity (dilated, diamond shape crypts). The surface epithelium show regenerative changes. |
Categories
All
|