|
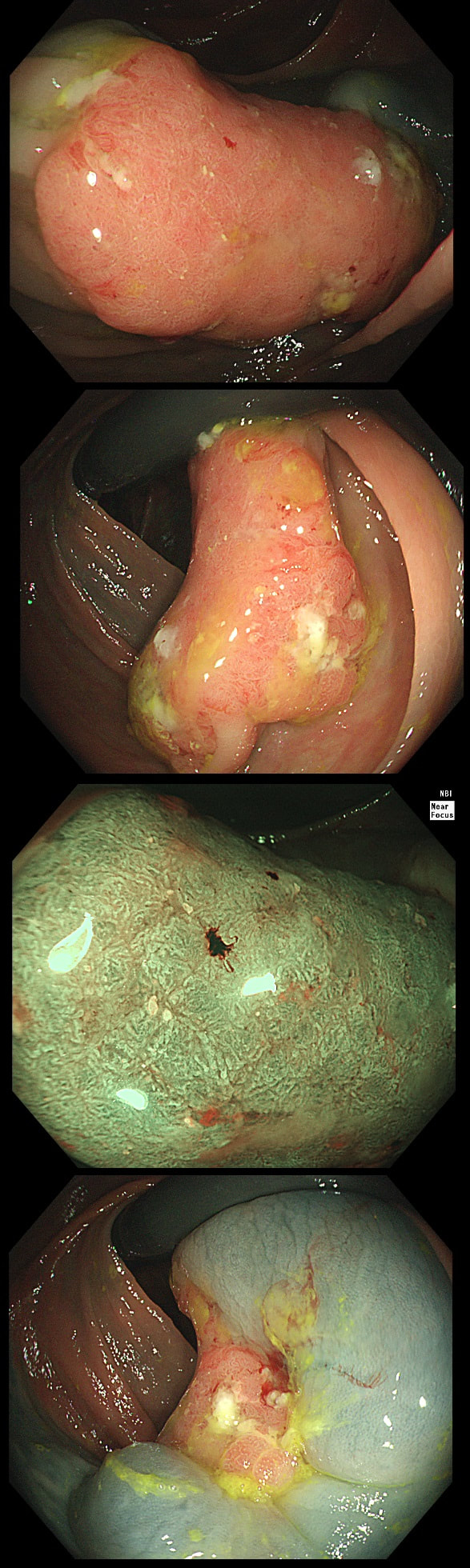
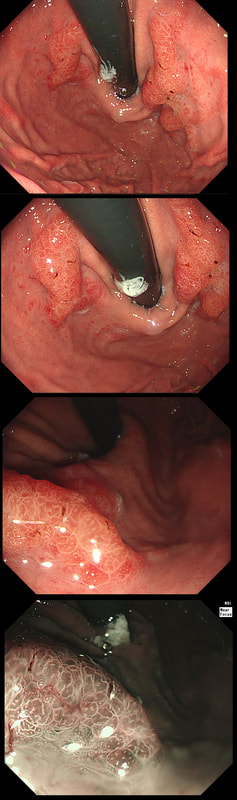
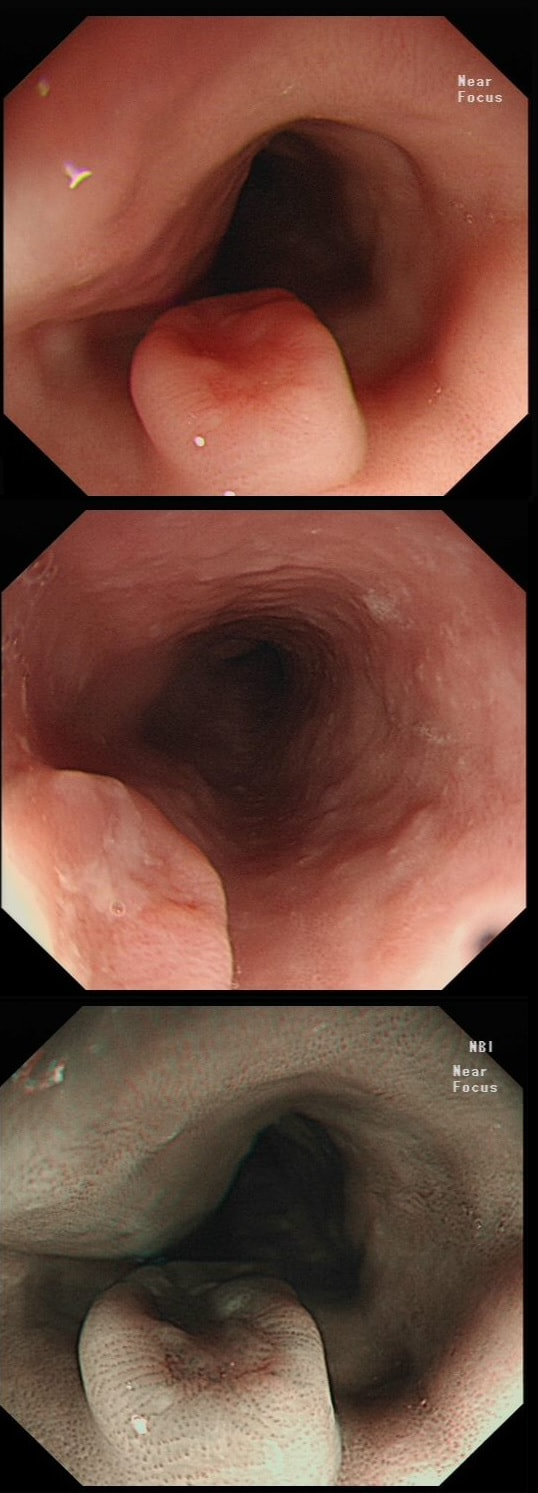
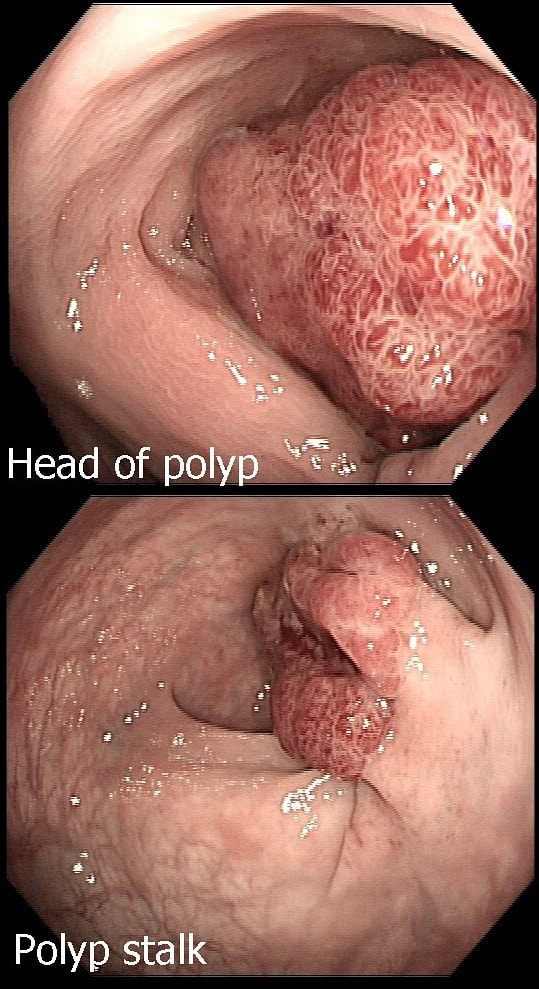
This patient is undergoing a gastroscopy because of dyspepsia. WHAT IS THE LIKELY DIAGNOSIS?
a) Hyperplastic polyp
It's unusual to see a ulceration on a hyperplastic polyp
b) GIST
Unlikely as the lesion isn't covered with entirely normal looking gastric mucosa
c) NET
Although there are none of the classical chunky vessels around its neck, there are other smaller nearby lesions with some unusually large vessels
d) Adenoma
Gastric adenomas are usually flat, plaque-like lesions
e) Early gastric cancer (EGC)
EGC is a good guess and patients with atrophic gastritis often do have nearby small type I gastric NET's. However, this isn't an EGC.
explanation
As you well know, gastric NET's are classified as; type I (70%-80% of gastric NET's) linked with hypergastrinaemia secondary to an atrophic gastritis and classically appearing as multiple, small gastric nodules. Then we have the rare type II gastric NET which account for about 5-8% is associated with hypergastrinaemia from a gastrin-secreting tumour such as in the MEN-1 syndrome or the Zollinger–Ellison syndrome. This was a type II gastric NET which has arisen in a patient with a pancreatic gastrinoma and MEN. Several other much smaller NET's have arisen in the nearby gastric mucosa. Finally, we have the type III NET (20%) which are solitary, large nodules with a high mitotic index arising in a healthy gastric mucosa. These are the ones not to miss as they need a cancer-like gastric resection. To remind you of the lessons from Prof Mark Pritchard's Podcast on gastric NET's, you should; AT ENDOSCOPY: • Look for atrophic gastritis • Consider using some pH indicator strips to measure the gastric pH (unless pt is taking PPI) • Identify all the NETs, record their size and number and sample them for histology and grading • Take antral and corpus biopsies and ask pathologist to do report on the presence/absence of gastric atrophy and intestinal metaplasia and also ask them to carry out immunohistochemistry stains for ‘gastrin’ in the antral biopsies and ‘chromogranin’ and ‘synaptophysin’ in the gastric body samples. • Look into the second part of the duodenum for the small submucosal gastrinomas which occasionally are seen in MEN-I • Consider samples for Coeliac disease if the patient has IDA CONSIDER OFFERING ENDOSCOPIC RESECTION FOR: • type I gastric NETs if >10-15mm • type II gastric NET if they’re causing problems (eg bleeding) and/or gastrinoma can’t be resected • type III gastric NET <1cm (provided that it's no worse grade 1/low grade 2 !) HISTOLOGY: If that proliferative index comes back surprisingly high (>10%), make sure that the pathologist hasn't inadvertently counted Ki67 positive cells in the nearby gastric mucosa. Atrophic gastric mucosa is usually more proliferative than the NETs! BLOOD TESTS: • FBC • Full haematinic screen including B12 and Ferritin of course • TFTs • Anti-parietal cell AB & Intrinsic factor AB titres • Serum gastrin level • Chromogranin level • Calcium and PTH level (particularly if MEN1 is suspected) REQUEST THE FOLLOWING SCANS FOR EVERYONE WITH LIKELY TYPE II AND III DISEASE: • CT • 68Gallium DOTA-peptide PET/CT scan • EUS to search for duodenal wall gastrinomas and small gastrinomas within the pancreas which CT can't see and to search for lymphadenopathy close to the NET
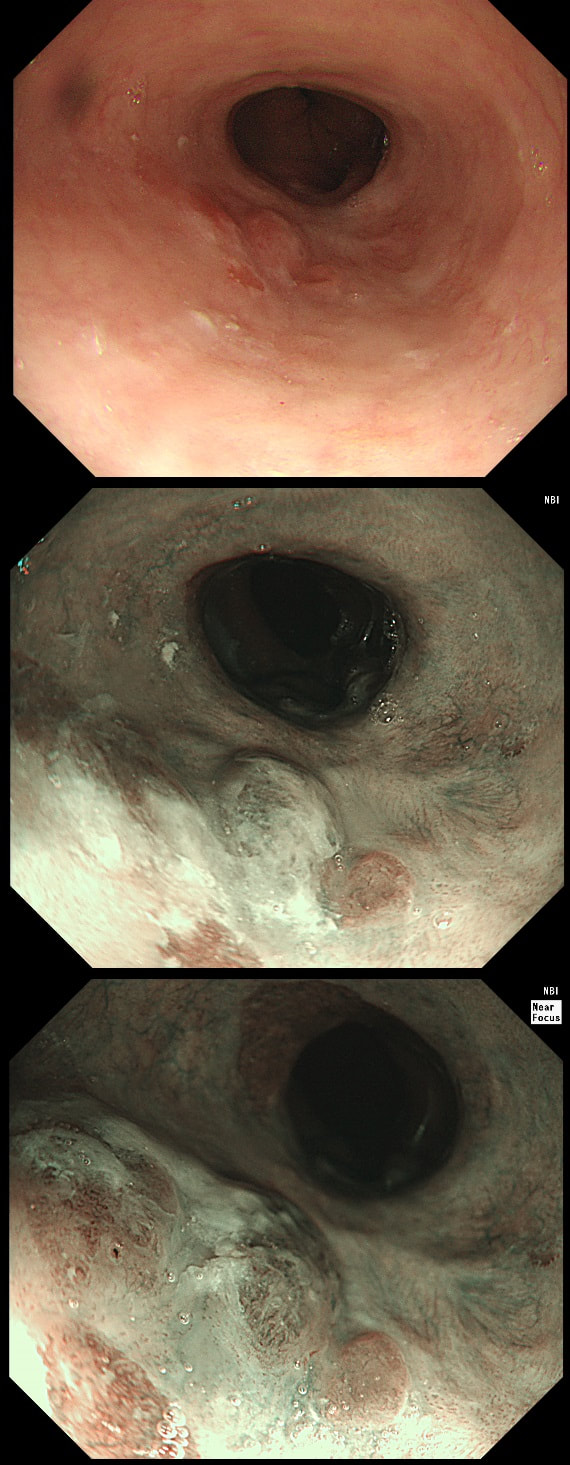
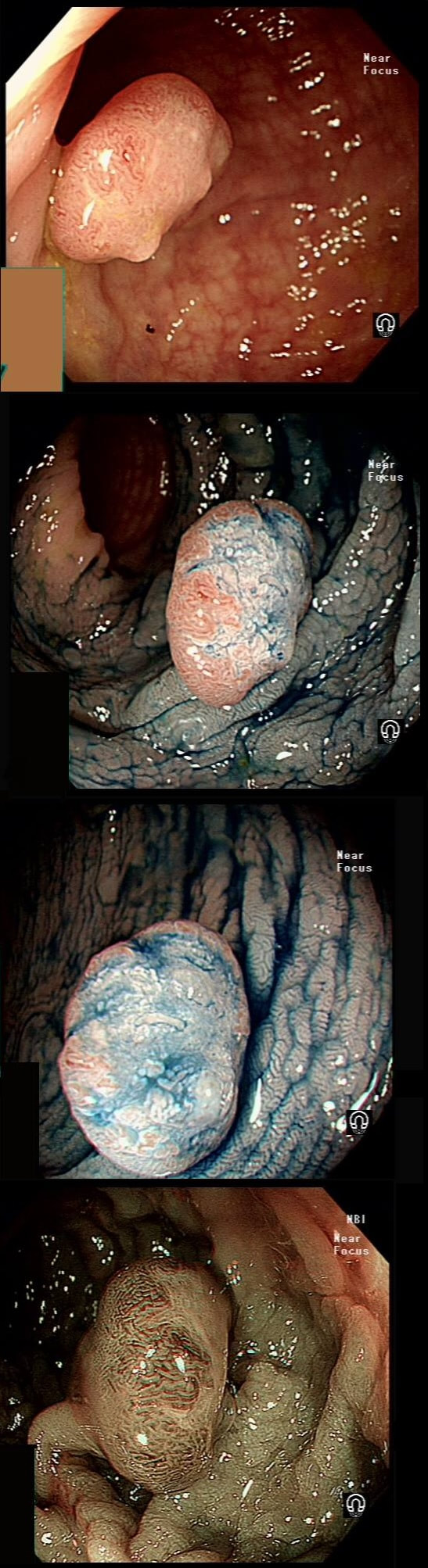
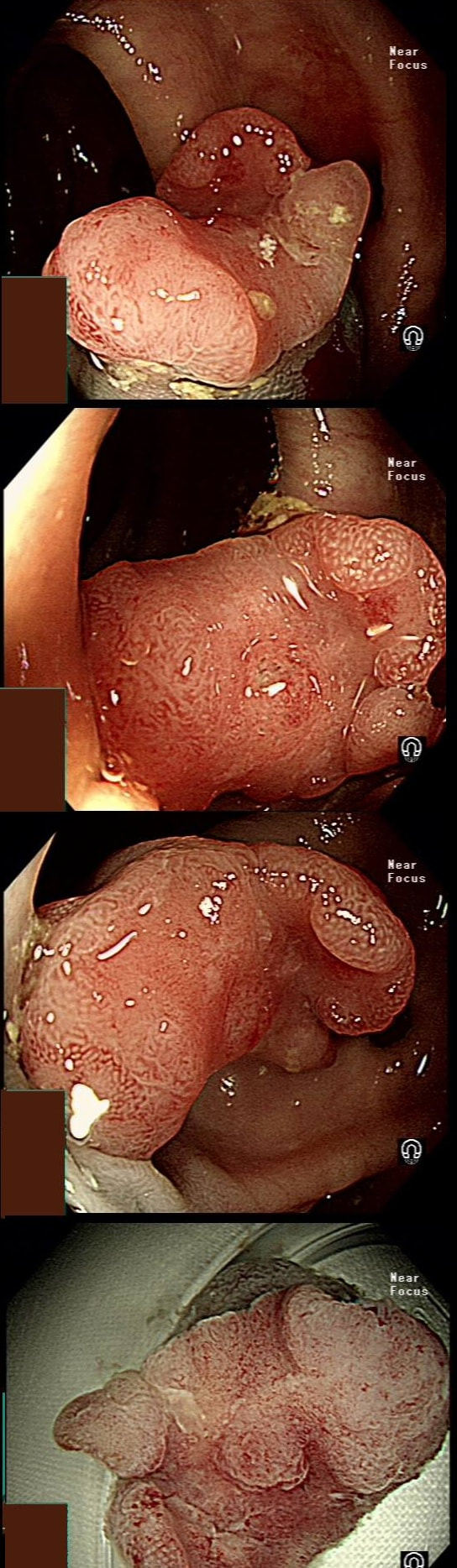
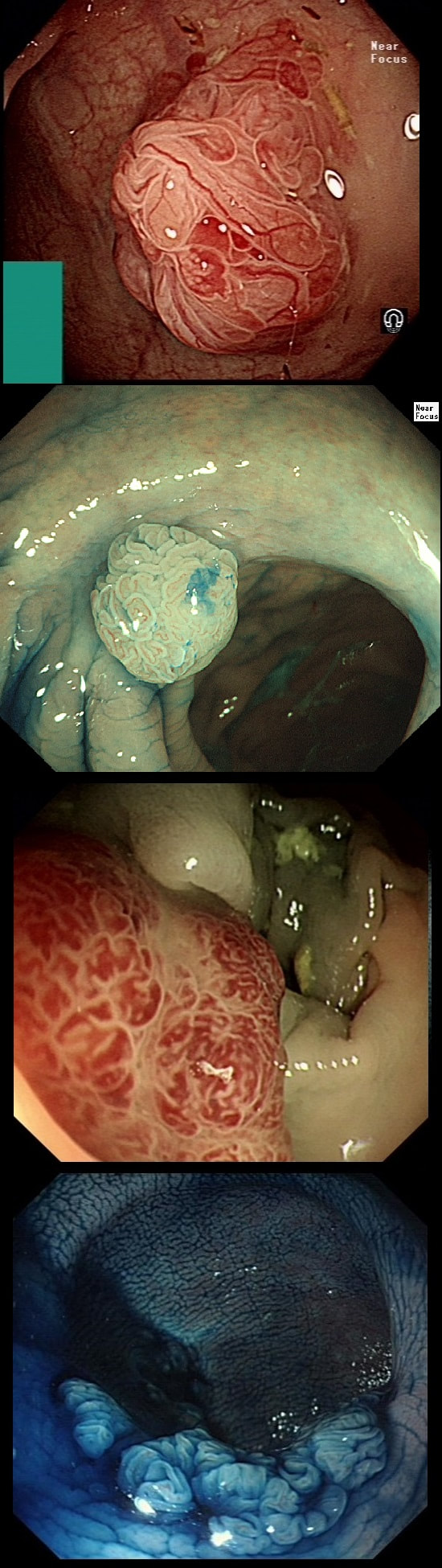
This lesion was found in the ascending colon and referred for removal.
WHAT IS THE LIKELY DIAGNOSIS?
a) HP
You must be joking!
b) SSL
You are not serious?
c) TA
Short slit-like crypts, well Yes but there is more to it!
d) TVA
Absolutely not!
e) Cancer
That non-lifting sign doesn't lie!
explanation
Must admit that I didn't like the look of this polyp. Sure, it does have an organised crypt pattern. I think that I can see short slits, making it a TA. This would fit with the fact that it probably is a LST-NG type of lesion (which are always TA's).
However, it also looks, 'chunky' and it's the 'thickness' of the lesion which made me suspicious. For this reason I was not surprised to find the non-lifting sign. As there is no lifting what-so-ever, I suspect that the lesion will turn out to be T2 at least.
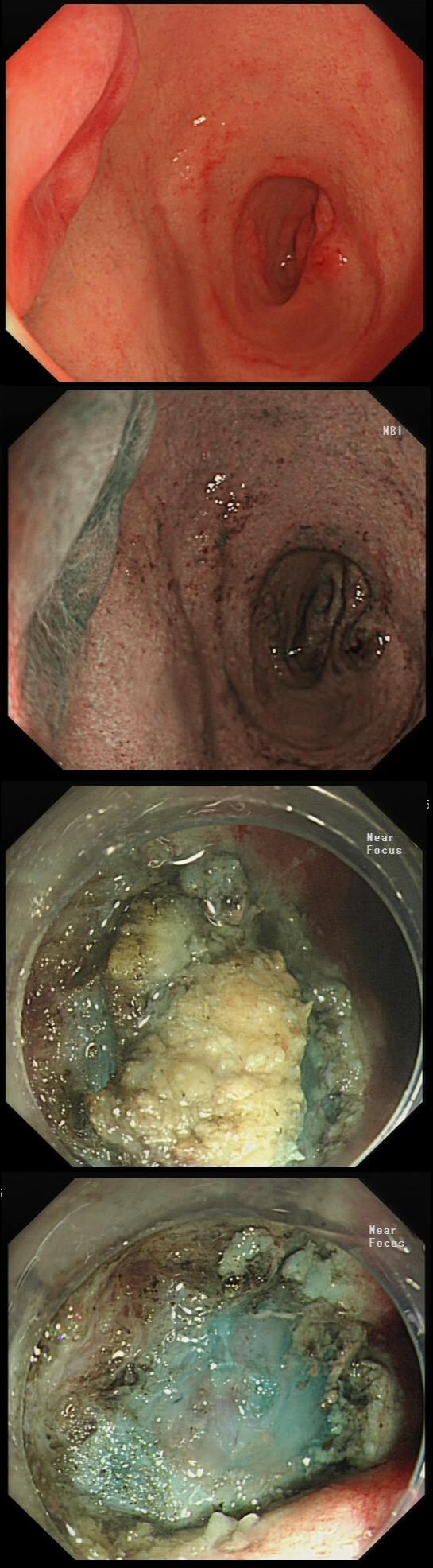
Patient with Barrett's harbouring HGD has been treated with RFA. He has now returned for the second RFA session when this is found.
WHAT IS YOUR ENDOSCOPIC DIAGNOSIS?
a) Benign scarring
Doesn't look benign - Looks evil!
b) Local recurrence of non-dysplastic Barrett's
Barrett's are re-emerging in several places. Doesn't look benign!
c) Barrett's adenocarcinoma
Absolutely! Nodules are never allowed in Barrett's!
explanation
Actually, there is a re-emergence of several brown nodules below the squamous mucosa. Biopsies confirmed this as an invasive adenocarcinoma, re-emerging from below the 'neo-squamous mucosa'. Clearly, to try RFA again would be a mistake! The histology showed a 'poorly differentiated' cancer and we are recommending either surgery or chemo-radiotherapy next (CRT). Even if histology hadn't shown poor differentiation, this 'smells' like bad disease to me which we may well 'undertreat' endoscopically.
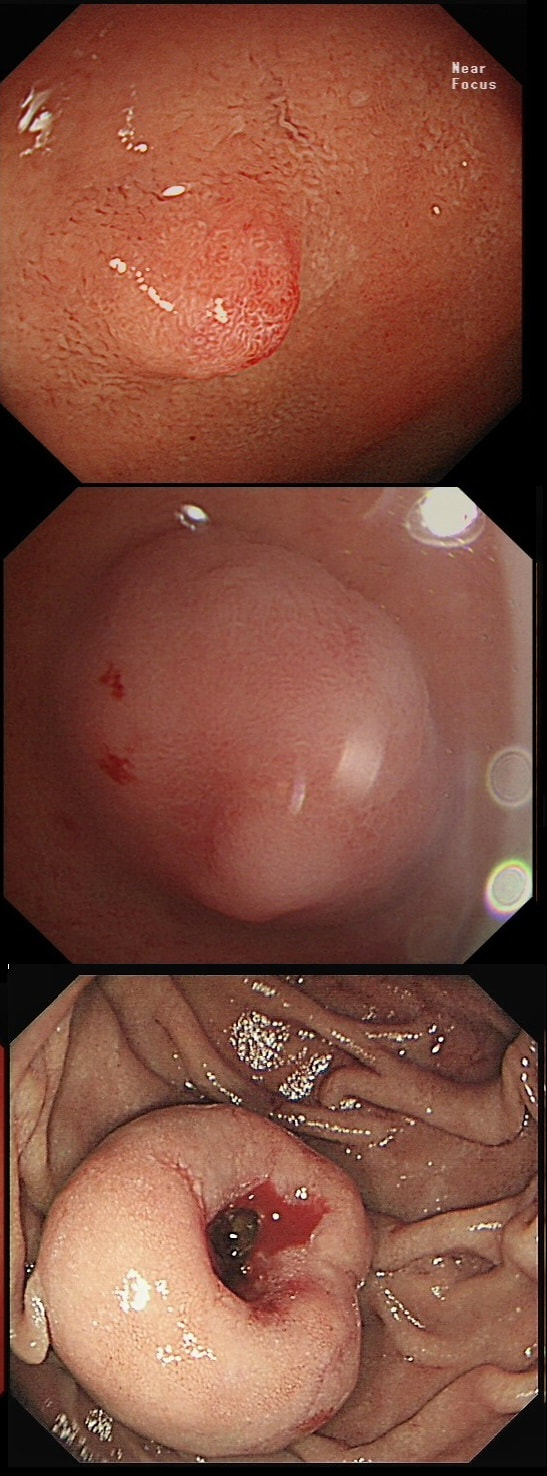
This is an interesting looking colonic polyp WHAT IS THE LIKELY HISTOLOGY
a) mixed serrated / adenomatous polyp
And that is what it looks like!!!
b) traditional serrated adenoma
But there are TWO components to this polyp!
c) serrated polyp undergoing malignant conversion
Well something has happened but 'lesion' has beautiful crypts!
explanation
Actually, the red bit of the polyp was composed of a tubulovillous adenoma and the rest was a serrated polyp! In other words, a mixed serrated/adenomatous polyp. Clearly the famous "three pathways to colorectal cancer": the APC mutation pathway (50%-70%); the mutator “Lynch syndrome” route (3%-5%); and the serrated pathway (30%-35%) can sometime get mixed up !!! This lesion, situated in a very spastic sigmoid, has been referred for resection. WHAT IS THE LIKELY DIAGNOSIS?
a) lesion is not neoplastic
Yes, hard to believe but true!
b) lesion is adenomatous
Lesion is 'battered' but crypts don't look typically adenomatous
c) lesion is malignant
No way, lesion isn't angry red, chunky or devoid of crypts!
explanation
I couldn't stop myself! After yesterday's case of a rectal 'prolapse polyp, part of the 'Mucosal Prolapse Syndrome', I had to show an example of a sigmoid mucosal 'traction polyp' (my nomenclature). The mucosa at the apex of this sigmoid fold is traumatised and inflamed but not actually adenomatous! Histologically these lesions also appear somewhat bashed up. This is where pathologists may see 'pseudo-invasion' which is actually movement of crypts due to trauma and inflammation. The sigmoid colon is the most 'powerful' part of the colon developing the force needed to go to the toilet. Presumably this is the reason that diverticular disease first develop in the sigmoid. The force can also create these pseudo-polyps from patches of inflammation which I presume gets tugged along with each peristaltic wave. The end result is that this is the most difficult part of the colon to make head an tail of polyps. These are common lesions and if you are a 'therapeutic endoscopist', you will be refer these lesions. In these cases, I don't go overboard by placing a snare far down the 'pseudo-stalk'. If you did, you will find that it's taking a long time to cut through all that healthy sigmoid mucosal fold and you run the risk of a perforation (early or late). Instead, I just catch the tip of the fold and ask my assistant to close the snare as quickly as possible. Of course, you don't need to worry about a type of chunky central vessel which you may find in an adenomatous polyp. Analysis of a small piece of mucosal apex confirmed a normal mucosa. Hopefully this was enough for everyone to relax ... This lesion in the low rectum has been referred for endoscopic resection WHAT IS YOUR DIAGNOSIS?
a) Mucosal prolapse syndrome
Well done!
b) Sessile Serrated lesion
Looks just like it but it isn't!
c) Traditional Serrated Adenoma
That's a No!
d) Adenomatous polyp
Patient complained of constipation...
e) Malignant polyp
But it's not 'chunky', red and it has crypts ?!?
explanation
There is no distinct edge to the 'polyp' and the crypts look normal, although a little splayed out and larger than normal, reminiscent of a serrated polyp. In real life, I had the advantage of knowing that the patient had been sent for colonoscopy because of constipation. I low-risk indication of course. There is no distinct edge to the 'polyp' and the crypts look normal, just a little splayed out and larger than normal. This 'polyp' is actually part of the "Mucosal prolapse syndrome", first described in 1983 by Du Boulay et al [J Clin Pathol. 1983;36:1264–8]. Obviously, this syndrome includes solitary rectal ulcers, inflammatory cloacogenic polyps but also rectal 'prolapse polyps' such as this. In addition, the 'Mucosal Prolapse Syndrome' includes those prolapsing mucosal folds in the sigmoid and perhaps most surprisingly - GAVE ! In a case like the above, a couple of superficial biopsies will usually reveal the true nature of the lesion as it's easy for pathologists to spot the fibromuscular hyperplasia with overlying epithelial crypt distortion rather than dysplasia. This lesion is being removed from the distal oesophagus WHAT IS THE LIKELY HISTOLOGY?
a) Hyperplastic polyp
You must be joking!
b) Adenomatous polyp
A gastric adenomatous polyp would surely now be malignant
c) Early malignant polyp
But it's arising from a malignant flat component!!!
d) Advanced gastric cancer
Absolutely!
explanation
Of course this is all very odd. Clearly this is an advanced cancer at the GOJ. What business do I have in 'attacking' this endoscopically?! Actually, the elderly patient had completed a course of chemoradiotherapy (CRT) for a T2, N0 junctional adenocarcinoma some 30 months previously. Now he has developed dysphagia and a CT confirmed a 2cm polyp at the gastro-oesophageal junction. Histology had shown 'at least IMca' and he was referred for consideration of an endoscopic resection. Clearly this lesion can't be cured endoscopically. In fact, the elderly patient is not a candidate for surgery and therefore there is no cure at all. However, I was thinking that as the cancer is mainly polypoidal, perhaps if the nodule could be removed, his swallowing will improve and he will not need a stent and could be offered brachytherapy. Clearly this was all 'speculative' but I'm glad to say that 6 months later, the patient still has not developed any dysphagia and his now starting brachytherapy. No doubt a better outcome than could be offered by a stent? This is a solitary gastric polyp WHAT IS YOUR ENDOSCOPIC DIAGNOSIS?
a) Hyperplastic polyp
Spot on!
b) Hamartomatous polyp
A bold guess but wrong!
c) Cystic fundic polyp
Too red and should be several of them!
d) Adenomatous polyp
But there is no defined crypt pattern?!
e) Malignant polyp
May be a 5% chance that you are right!
explanation
A missing piece of information, which I perhaps should have provided, was the H.pylori status of this patient! This lesion was arising from a H.pylori associated gastritis.
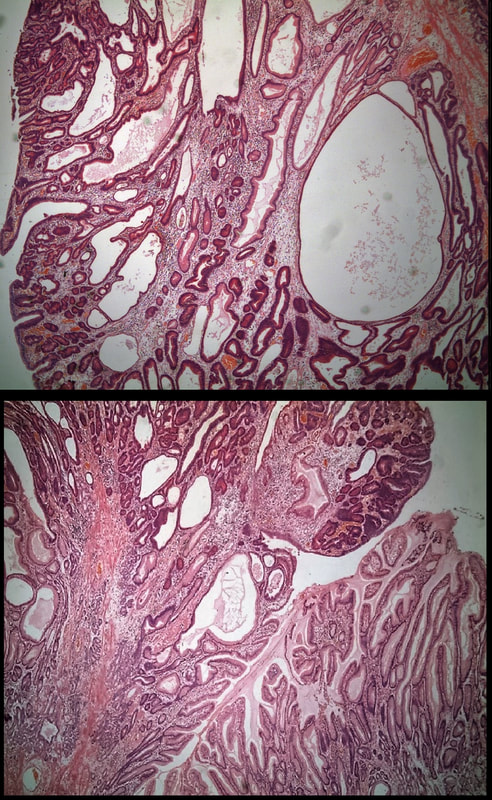
Most polyps found in a stomach with Helicobacter pylori associated gastritis are hyperplastic (inflammatory). They appear angrily red (because they have lots of capillaries) and often with white fibrin caps, making them look a little like mushrooms. A small proportion of hyperplastic looking polyps are actually malignant. In my experience, these 'stealth cancers' are most common close to the gastric cardia and are always solitary. Conversely, multiple inflammatory polyps in the antrum and gastric body are almost certainly benign. Of course, this is a 'solitary' hyperplastic polyp, close to the fundus. Perhaps not unexpectedly it harboured some (low grade) dysplasia (histology below). The dysplasia is in the crypts lined by deep purple/blue cells rather than the normal light pink cells. The contrast is probably best seen in the second histological slide where the dysplastic part is towards the top of the slide and the non-dysplastic is at the bottom half. If the H.pylori test is negative, and particularly if the patient is taking a PPI, the polyp is more likely to be a 'Fundic gland polyp' (cystic fundic polyp) which are full of cystic spaces and therefore look a little translucent like frog-spawn. Rarely polyps are hamartomatous as in Peutz-Jeghers polyps and the polyps arising in patients with 'Cronkhite-Canada syndrome or in Juvenile polyposis. In many cases these lesions have an odd but distinct surface crypt pattern or, alternatively look again look translucent like frogspawn. It's rather difficult to explain the appearance of a hamartomatous polyp and for this reason, I have attached some images below (after the histology slides). Another polyp, most likely to arise in an atrophic gastritis with patches of intestinal metaplasia, is an adenomatous polyp of the 'intestinal type'. To remind you, there are at least 4 types of gastric adenomas which all have an organised and regular surface crypt pattern:
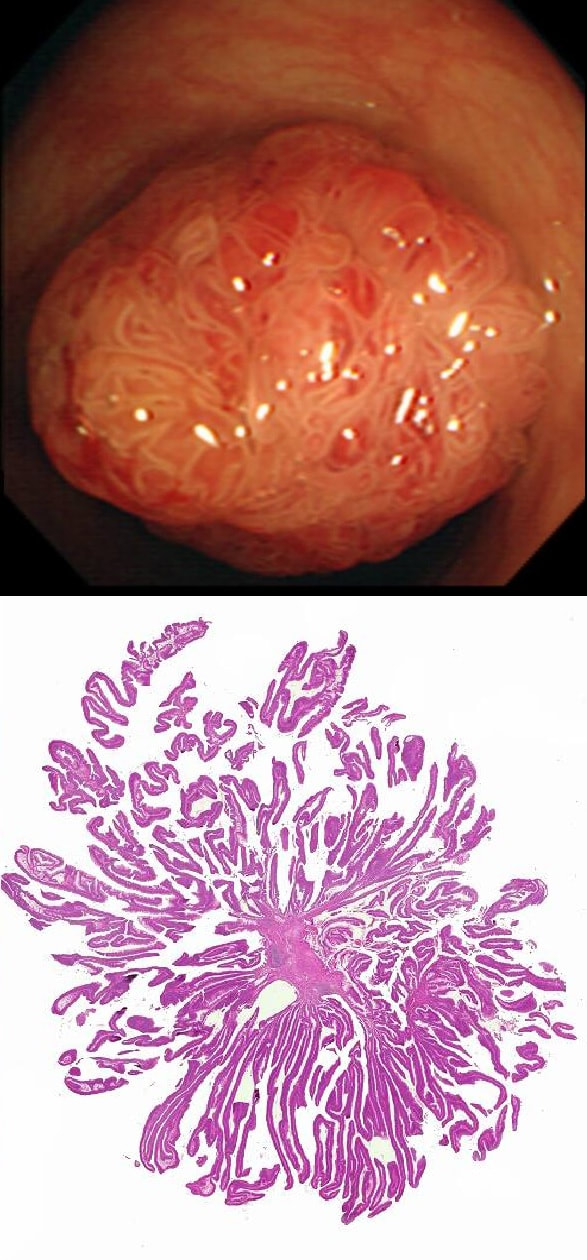
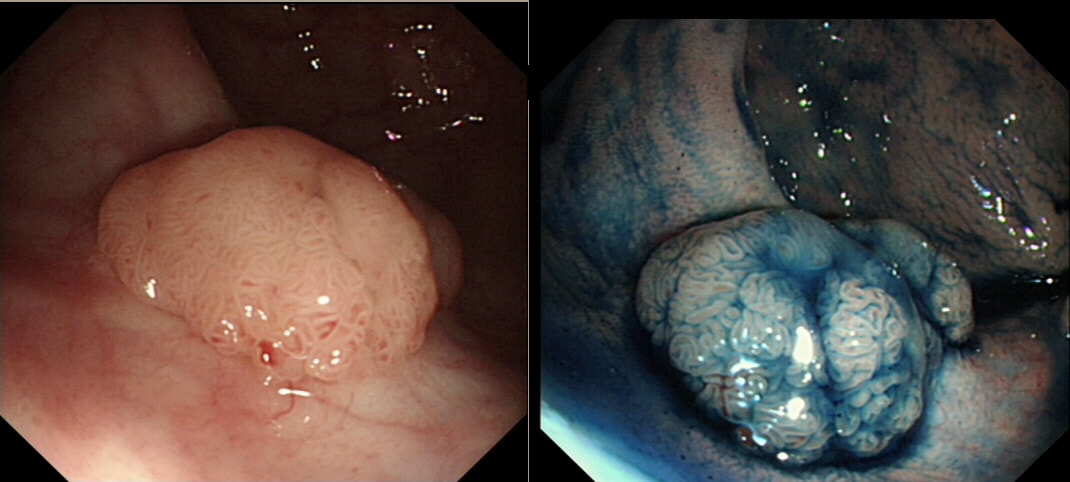
This polyp is pretty when viewed by the endoscopy but stunning below the microscope!
WHAT IS THE HISTOLOGY?
a) Tubular adenoma (TA)
This polyp is prettier than that!
b) Tubulovillous adenoma (TVA)
Endoscopically it could be but histology says otherwise!
c) Villous adenoma (VA)
Absolutely!
d) Traditional Serrated Adenoma (TSA)
Endoscopically Yes, but histologically No!
e) Sessile Serrated Lesion (SSL)
Would be unusal for an SSL to be 'subpedunculated'
explanation
Endoscopically it can be difficult to recognise a villous adenoma. Unless you submerge the lesion to see the villi rise up like the arms of a sea anemone. Apparently, they are quite difficult to 'process' by the pathology lab as well. They are fragile and those beautiful villi, easily become damaged.
Of course, villous adenomas are regarded as 'high risk lesions'. The other high risk findings often used as surrogate markers for cancer in guidelines are: having 3 or more adenomas, polyps 10mm or larger, and polyps harbouring high-grade dysplasia. However, if you have to make a choice of the two strongest predictors of future cancer risk, a study in Gastroenterology found that it would be; finding a polyp 2cm or larger and resecting a polyp found to harbour HGD [Gastroenterology 2019;158(4);875-83].
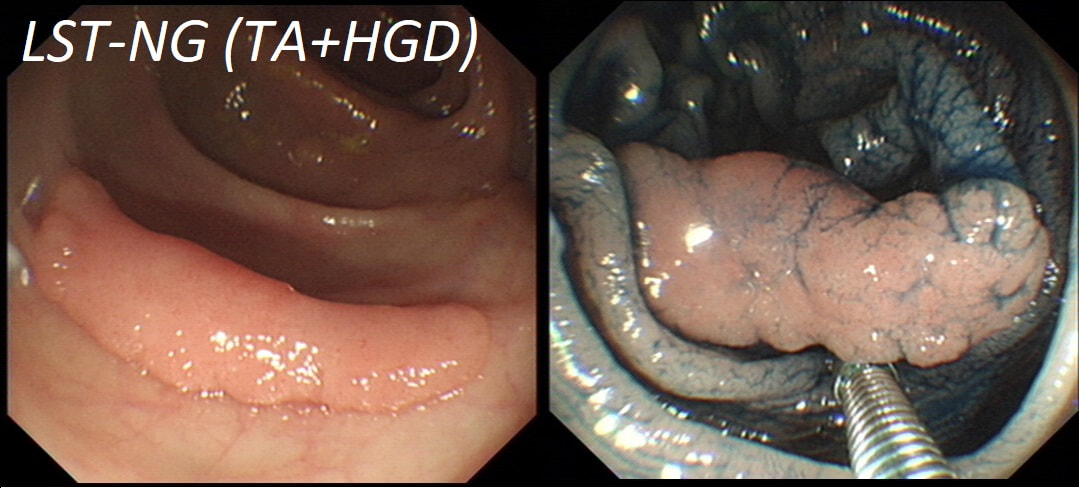
A polyp found in the transverse colon
WHAT IS YOUR ENDOSCOPIC DIAGNOSIS?
a) Tubular adenoma
You are half correct!
b) Tubulovillous adenoma
On the left side, the crypt's are slit-like rather than gyrate
c) Villous adenoma
VA's are soft like sea anenome !
d) Serrated polyp
No, the crypts are not large, round openings!
e) Malignant polyp
Yes, there are no crypts on the right-hand side !
explanation
Did you spot that cancer has developed on the right side of this tubular adenoma (TA). On the left side, the crypts are slit-like whilst on the right hand side, the crypt pattern has been destroyed (Kudo type V crypt pattern). The Haggitt stage was I and I took particular care in removing the polyp with a large margin. The cancer was only 4mm in diameter. Perhaps the smallest adenocarcinoma of the colon you will ever see ?
This is the scar following the piecemeal removal of a sigmoid polyp some 6 months ago. It was a 15mm tubular adenoma harbouring high-grade dysplasia. Samples from the EMR scar has showed 'distorted glands' only.
WHAT WOULD BE THE CORRECT FOLLOW UP?
a) organise an immediate follow-up
WTF ! This not a normal scar! You absolutely need to organise more samples, and perhaps a CT !!!
b) organise a follow-up in 6 months time
Would have been a mistake !
c) organise a follow-up in 12 months time
Would have been a big mistake!!!
d) organise a follow-up in 3 years time
May have cost the patient his life!!!
explanation
The first EMR was piecemeal and histology could of course not confirm that the resection had been complete. Indeed the 'index histology' reads oddly mentioning "frequent mitotic figures" and "back to back glands". To a gastroenterologist these words does not sound particularly alarming.
However, the pathologist was trying to say "this looks like cancer but I can't actually make that diagnosis" !!! Indeed this doesn't look like a normal EMR scar! The whole area is indurated as if there is an infiltrative process below the mucosa. Histology was reassuring mentioning some distorted crypts only. Sadly, the endoscopist was content with the reassuring repeat histology and did not reflect on the worrying endoscopic appearances. He did NOT organise a second round of post-EMR samples and the patient returned 2 years later with an advanced cancer. The take home messages from this sad story?
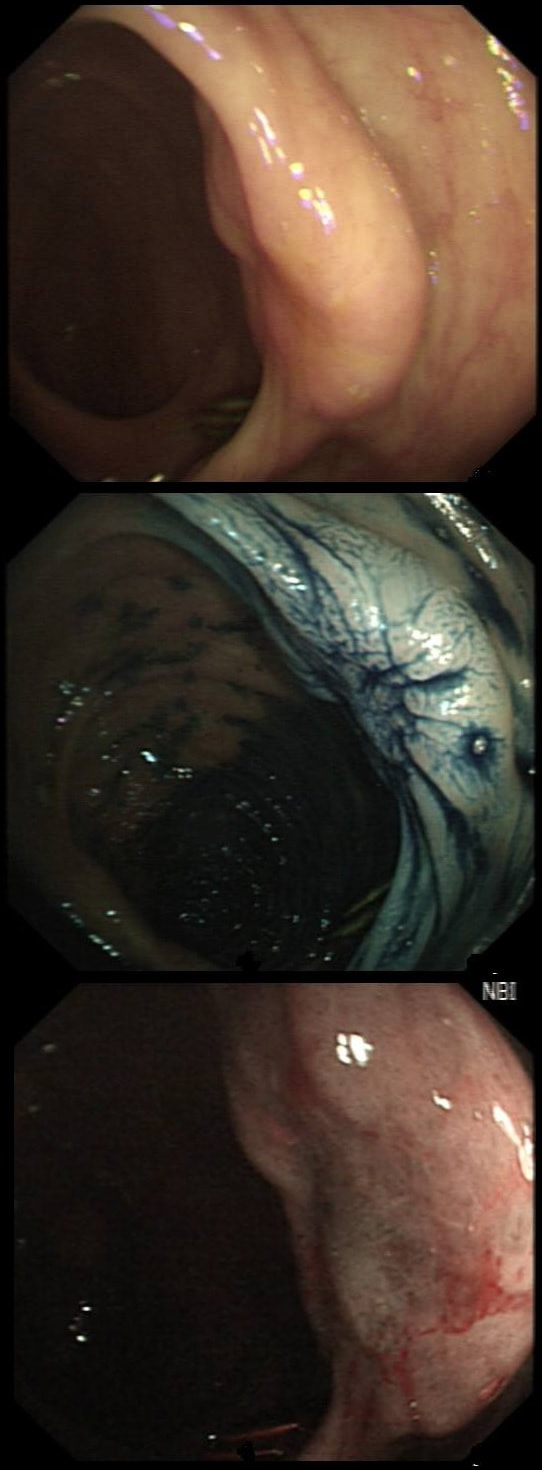
An odd lesion in the middle of the oesophagus of a 55 year old woman. WHICH OF THESE POSSIBILITIES IS THE MOST LIKELY?
a) Papilloma
Papillomas look more warty and I've never seen them ulcerate
b) Leiomyoma
Statistically the most likely benign lesion probably!
c) GIST
Are very rare in the oesophagus!!!
d) NET
Are also very rare in the oesophagus!
e) SCC
Not likely as the IPCL's are regular and the lesion isn't brownish!
explanation
GIST's are extremely rare in the oesophagus whilst common in the stomach. In contrast, leiomyomas are common in the oesophagus and rare in the stomach. However, this looks odd and the previous biopsies had not been able to get to the bottom of the diagnosis. Are we missing a SCC? However, it looks like something from deeper layers were breaking through the mucosal surface. In contrast an SCC should start growing at the surface and in time invade deeper. The 'intrapapillary capillary loops (IPCL's) dilate up, become irregular and eventually completely disorganised in SCC's. At the same time the oesophageal wall takes on a brownish tint on NBI.
I decided to remove it and it turned out to be a leiomyoma. I presume that it had got traumatised or perhaps by pressure necrosis ended up with a funny surface. Don't think that I have seen an oesophageal NET as yet. Presumably they are very rare in this location. By the way, I've uploaded an example of a T2 mid-oesophageal SCC in the clip below. The lesion looks smallish but is already beyond endoscopic cure and Lugol's shows up a large area of background dysplasia which looks orange rather than brown after Lugol's dye spray.
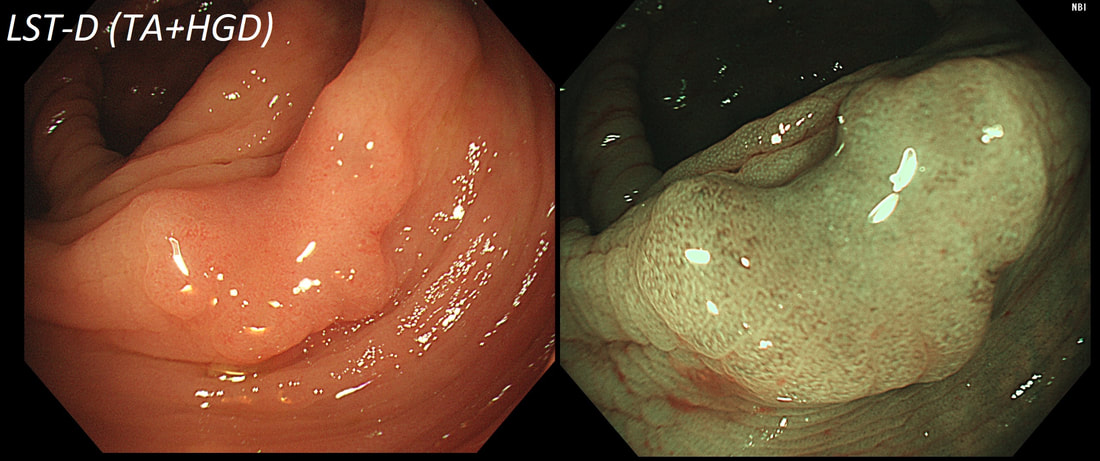
This polyp on a short stalk was removed from the colon
WHAT IS THE LIKELY HISTOLOGY?
a) TA
At the edge, the crypts are clearly sli-like but how about the centre?
b) TVA
Doesn't look like gyrate crypts!
c) VA
Doesn't look like a sea anenome!
d) TSA
TSA's do have crypts but this lesion doesn't in the centre!
e) Early cancer
Was my own firm diagnosis!
explanation
The head of the lesion is clearly of concern. There are 'horns' on it! Of course, the devil has horns but as it was arising from a stumpy stalk. I went ahead and removed it. Somewhat surprising our pathologists called the lesion TA+HGD!
Admittedly, there was disagreement between our histopathologists and 2 out of 5 believed that it was an invasive cancer. Endoscopically, the lesion is clearly malignant and at the very least it's an intramucosal cancer (IMca). Of course, our UK histopathologists are unable to make the diagnosis of Intramucosal cancer in the colorectum because this is not a diagnosis recognised by the 'Vienna classification'. Elsewhere in the GI tract, intramucosal cancer is a diagnosis which our pathologists are 'free' to make. It makes no sense whatsoever to me !
This lesion was referred for resection just beyond the pyloric ring. It seemed to lift well but rather to my surprise, the snare cut through the base of the lesion (bottom image on the left). It took some further lift and a stiff snare to scrape the lesion of the muscle propria layer.
WHAT IS THE LIKELY HISTOLOGY?
■ Adenoma
Adenomas are usually not umbilicated ...
■ Brunners gland hyperplasia
I don't bother to remove those and they are not umbilicated!
■ Gastric heterotopia
Is usually found at this location but never require EMR
■ NET
Of course it is - it's umbilicated !!!
■ Early malignancy
Would be VERY rare in the duodenal cap!
explanation
The lesion is umbilicated... As you probably remember, umbilicated lesions in the duodenum are usually NET's. For some reason, they look different to NET's found elsewhere in the gut, where thin vessels are usually seen crawling up their sides. In the photograph below, you can see that at the very earliest stage (first image), there is no central indentation. However, very soon a subtle central dip develops (second image) which at the advanced stage (when I was tasked with removing the lesion), it has a deep central pit which was intermittently bleeding.
This proved to be a WHO grade I NET (neuroendocrine tumour, with a mitotic rate <2%). These lesions always seem to be tethered down close to the muscle propria and can be difficult to remove without taking a chunk of muscle propria with it. Duodenal NET's should of course be discussed at the 'neuroendocrine MDT' and usually end up having: 1) Measurement of plasma chromogranin A (pCgA) levels. CgA is produced by all cells derived from the neural crest and high levels are found particularly in patients with metastatic disease. 2) A ‘gut hormone screen’ (measurement of gastrin, glucagon, vasointestinal peptide, somatostatin and pancreatic polypeptide levels). 3) Finally, an ‘octreotide scan’ (somatostatin receptor scintigraphy) or, even better, a PET/CT scan using peptides that bind to somatostatin receptors (68Gallium-DOTA-TOC/NOC/TATE).
This is an odd looking sigmoid polyp which is seen arising from a broad stalk.
WHAT IS YOUR ENDOSCOPIC DIAGNOSIS?
■ Tubular adenoma (TA)
But the crypts don't look slit-like do they?
■ Tubulovillous adenoma (TVA)
Was my first thought but it doesn't look quite right...
■ Villous adenoma (VA)
If your bx forceps disappears into the polyp it's a VA
■ Traditional Serrated adenoma (TSA)
You'd be excused if you got this wrong - these are rare beasts!
explanation
My initial endoscopic diagnosis was that of a TVA. However, it looked a little "exaggerated". Actually it turned out to be a "traditional serrated adenoma" (TSA).
TSA's are rare and mysterious polyps. They only accounting for about 1% of colorectal polyps and are most common in the sigmoid and rectum. Endoscopically they can appear as “exaggerated” tubulo-villous adenomas or as villous adenomas. In the image below, I've put four examples of TSA's together to illustrate how differently these can appear ! It has been proposed that because one-third of "serrated cancers" are found in the distal colon and perhaps these cancers arise from TSA’s! However, genetically, TSA's are more like adenomatous polyps harbouring low-level microsatellite instability (MSI-L). They usually contain KRAS mutations rather than the BRAF mutations which you find in the 'Sessile Serrated Lesions'. For this reason, it's perhaps unlikely that they contribute to the 'Serrated Pathway' to cancer. By the way, a recent paper has highlighted that patients with TSA's are also at greater risk of 'high risk polyps', elsewhere in the colon.
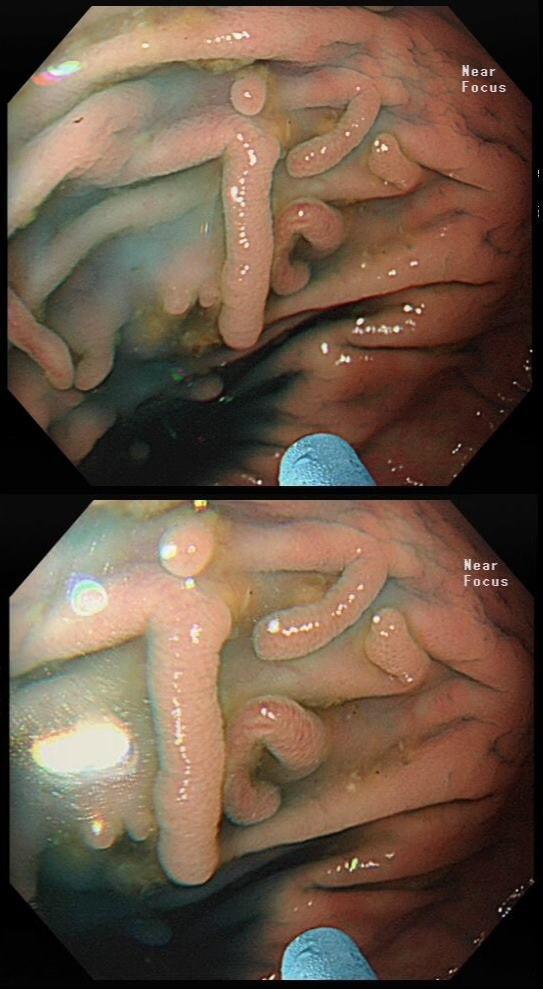
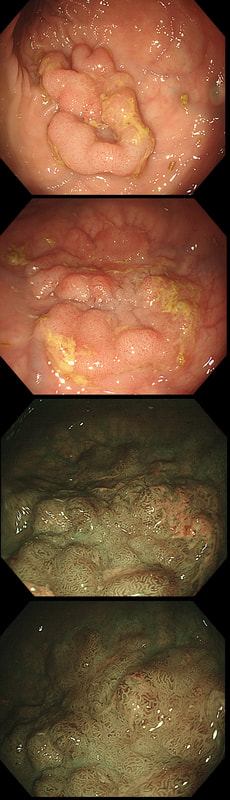
These peculiar colonic lesions were found in the colon of an asymptomatic patient.
WHAT ARE THEY?
■ Inflammatory polyps
Absolutely!
■ Adenomatous polyps
These are too long and peculiar looking to be adenomatous!
■ Hamartomatous polyps
No way!
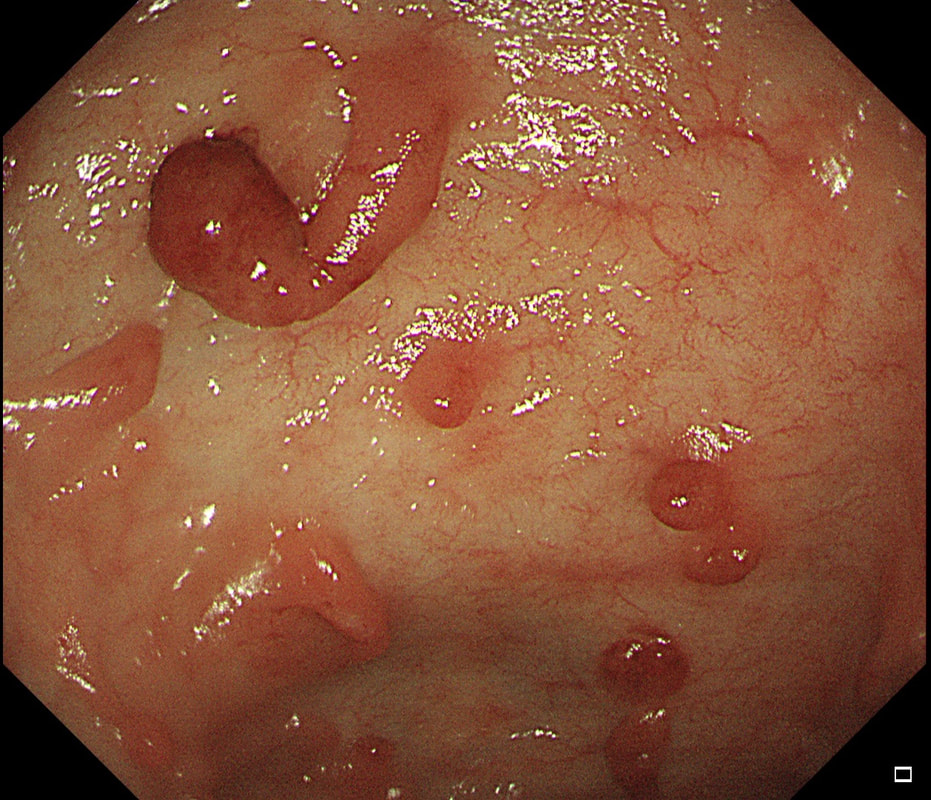
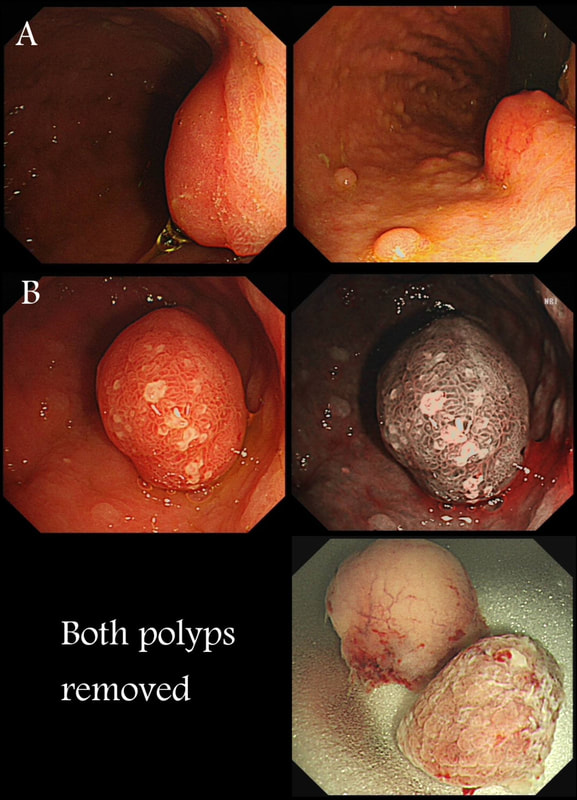
Two gastric polyps (labelled A & B) were both removed from the stomach of a 60 year old woman who complained of indigestion.
WHAT IS THE LIKELY DIAGNOSIS?
■ Both are hyperplastic
But the look different to each other!
■ NET + HP polyp
Well done ! And presumably you know which is which?
■ Both are NET's
But they look different!
■ HP + Adenoma
How about those fine vessels seen in the second image?
■ Both are adenomatous
But they look different!
explanation
You can see the thin vessels crawling up the side of polyp A. This appearance is typical of a gastric NET. Polyp B has a more villous surface with a few white spots - all typical of a hyperplastic gastric polyp. Some of these may contain some malignant cells which somehow generate a hyperplastic/reactive/inflammatory reaction around themselves. However, most hyperplastic gastric polyps are benign and arise secondary to a Helicobacter pylori gastritis. For this reason, you should always do a CLO test in these cases.
Every few weeks, I look up the notes on Prof Pritchard's' Podcast on gastric NET's to remind myself of the workup of these cases. As you remember, you should take samples to confirm an atrophic gastritis whenever you find a gastric NET. In this case, the patient did NOT have an atrophic gastritis. Instead, there was a Hp associated gastritis which is the reason for the second polyp. We realised that what we had was a TYPE II gastric NET! Analysis confirmed that the 17mm was WHO grade II. The finding of anything else than an innocent Type I gastric NET means that further imaging was required. A little late in the day of course but fortunately, the following imaging investigations were unremarkable:
Anyway, below is a reminder of what to do at gastroscopy, when you have a case of gastric NET:
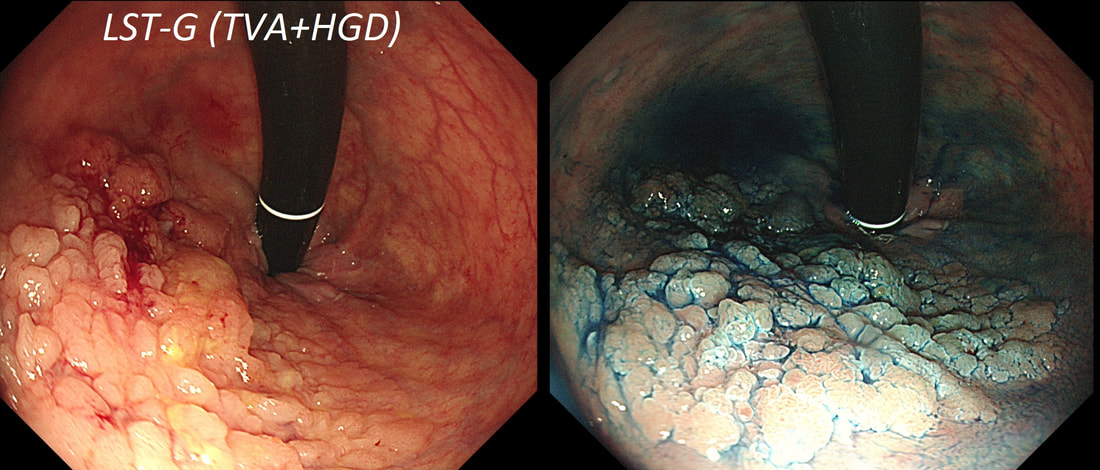
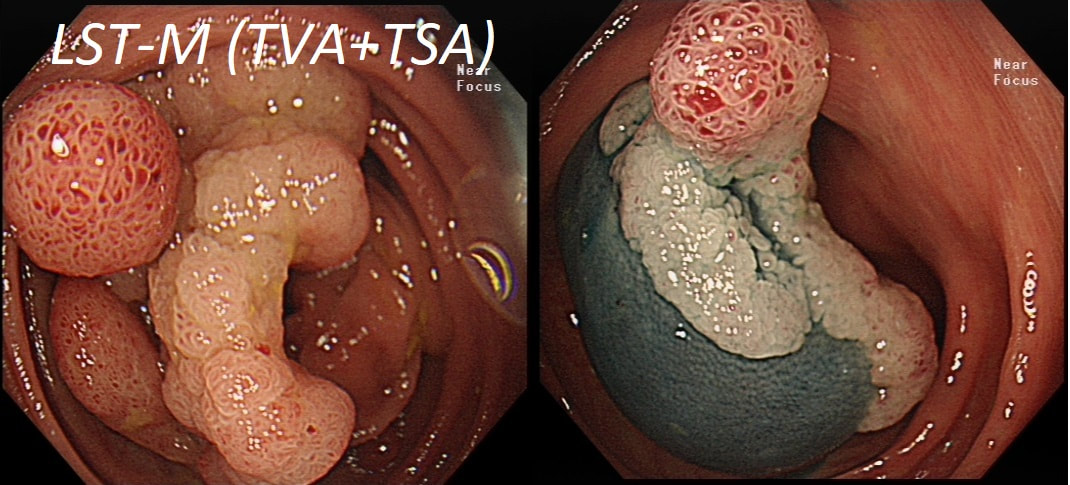
This lesion was found in the ascending colon.
HOW WOULD YOU DESCRIBE IT?
■ Flat elevated lesion (IIa)
Only lesions 10mm or smaller are referred to as 'IIa lesions'
■ Flat elevated lesion with a central depression (IIa+IIc)
But that depression dissappear when lumen is inflated!
■ Laterally spreading tumour of the non-granular type (LST-NG)
But the surface is 'cobbled' not smooth!
■ Laterally spreading tumour with a central depression (LST-D)
LST-D's are always TA's (and therefore have a smooth surface)
explanation
Lets just state something from the start! A flat elevated polyp is of course a IIa type of lesion BUT when larger than 10mm in diameter is no longer referred to as a IIa lesion. Now, it's a 'Laterally Spreading Tumour'!
There are 4 types as follows:
In this case the crypts are gyrate, (type IV crypts), making it a TVA. Laterally Spreading Tumours with a gyrate crypt pattern are all TVA's and almost never harbouring more than LGD. OK but it looks depressed in the centre doesn't it? No, it isn't depressed in the centre! It's simply they way the large lesion has folded itself. This is something of a 'trick question' as I deliberately didn't inflate the colonic lumen fully in the first image. When the lumen is inflated, you can see the true shape of the lesion; a LST-G ! A meta-analysis in 2013 by Voorham et al. [Voorham QJM. AM J Gastro 2013;108(7):1042-] concluded that pedunculated polyps were more likely to harbour KRAS mutation and APC mutations than flat lesions, and that flat lesions were more likely to harbour BRAF mutations. Depressed lesions and LST-NG’s were particularly unlikely to contain KRAS. Below are some examples of LST's This sizeable polyp was discovered at the ileo-caecal valve and was referred for resection. Samples have not been taken to avoid tethering down making the endoscopic resection uneccessarily difficult. HOW WOULD YOU APPROACH THE LESION?
■ Back off and take some samples
Yes, and perhaps request a CT!
■ Attempt an EMR
You first need to decide what this is!
■ Consider removal by ESD
Can't be adenomatous, it's growing out of the TI!
■ Underwater EMR
Would also be ill advised
■ Refer for surgery
If this is what you think it is, then Yes!
explanation
In most cases, I would say 'if it lifts it will shift'. However, in this case I wouldn't bother with a test-lift. The reason is that the thing is growing out of the terminal ileum. You don't get adenomatous polyps growing out of the TI! This must be something else! In fact the true lesion may be larger than the red nipple-like polyp. Even though there are no large 'tell-tale' vessels running up it's side, the only thing of this size, growing out of the TI, is a NET! A lymphoma was my second guess. TI NET's are often bad news and should be considered for surgical resection. There is another odd thing about NET's growing in the terminal ileum. The WHO grading system doesn't seem to relate to the aggressiveness of the lesions behaviour ! This was only WHO grade I (proliferation index was only 1.8%) but on a full analysis after the right hemi-colectomy, the NET was found to be a invading into the muscle propria layer and with metastatic deposits in 2 out of 12 resected lymph nodes (T2,N1) as well as ulcerated deposits of NET in the nearby pericolic fat on the serosal surface. The moral of the story? In the terminal ileum, 'well differentiated NET' doesn't mean that it's well behaved!
This lesion was found in the rectum of a patient undergoing colonoscopy because of constipation
WHAT IS THE LIKELY DIAGNOSIS?
■ Polyp from mucosal prolapse
Clever!
■ Serrated polyp
Crypts look a little serrated but the rest doesn't
■ Adenomatous polyp
Polyp doesn't look 'neoplastic'
■ Malignant polyp
Now, this doesn't look malignant!
explanation
Well perhaps the crypts look a little like serrated crypt openings but somehow the rest of the polyp doesn't look like a typical serrated lesion. Where is the covering mucus?! Furthermore, the polyp definitely doesn't look adenomatous or malignant!
Actually this has arisen as part of the 'Mucosal prolapse syndrome' which is the umbrella term for entities such as; solitary rectal ulcer syndrome and inflammatory cloacogenic polyps. Patients are often constipated or have difficulty with defaecation with straining on the toilet or undergo the sigmoidoscopy because of tenesmus, altered bowel habits or incontinence. Surprisingly, some patients don't have any straining-related complaints! Most pathologists would recognise the typical mild fibrosis, thickening of the muscularis mucosae and crypt irregularity (dilated, diamond shape crypts). The surface epithelium show regenerative changes.
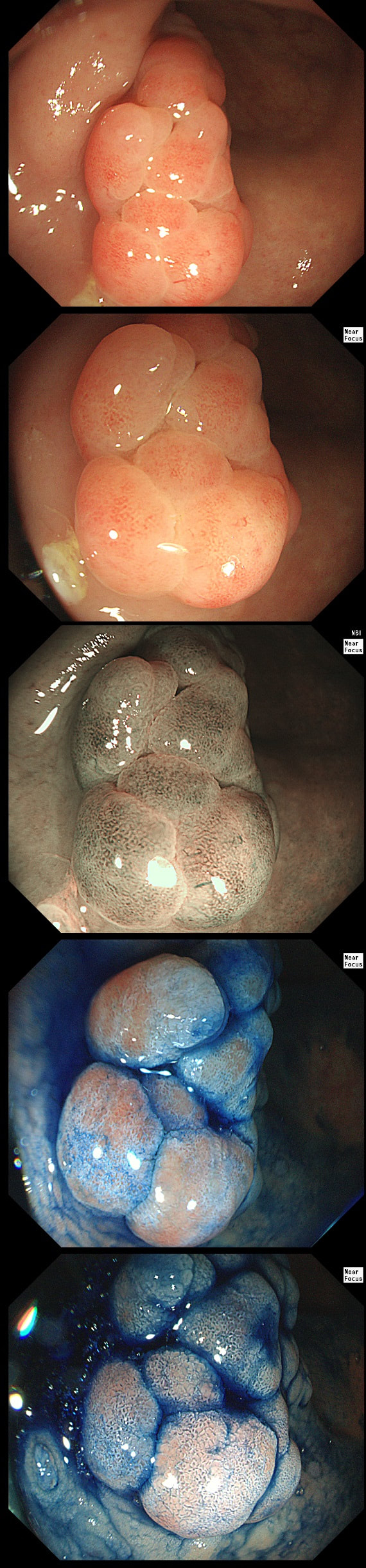
This polyp was found in the sigmoid.
HOW IS IT BEST REMOVED?
■ Cold snare polypectomy
Wouldn't be my first thought!
■ EMR
I agree - single fragment EMR!
■ ESD
Would just add time and expense to procedure (unless you need practise, I guess)
explanation
This 10-12mm polyp is covered with a beautiful gyrate crypt pattern typical of a TVA. The risk of cancer deep within, in spite of the normal surface is far below 1%. But it's not 0% and it would also be a little difficult to cut through this amount of tissue. For these reasons, I think that 'cold snaring' would be wrong.
At the other end of the spectrum, we have an ESD. It would ensure a single fragment resection but would take about 20 minutes or so. The in between the two, we have an EMR. If course, a single injection of 3-4ml would lift this nicely and then a 15mm snare would resect it, single fragment, within a minute. I think that the best method of removal of polyps up to about 2cm, is by EMR as polyps up to this size can usually be removed as a single fragment. This is an elderly patient undergoing an OGD because of IDA (iron deficiency anaemia). A lesion catches my eye on the anterior gastric wall WHAT IS THE LIKELY HISTOLOGY?
■ Gastric xanthelasma
The colour is suggestive and gastric atrophy would be the link but it's not quite right...
■ Healed GU scar
But how about that funny crypt pattern?
■ Gastric adenoma
Yes, doesn't this look like a tubular adenoma?
■ Early gastric cancer
Wouldn't the crypt pattern then be very disorganised or perhaps very small?
explanation
The pale colour is odd and reminiscent of gastric xanthelasma which as you know is linked with gastric atrophy which is the likely cause of this patients IDA. However, when I zoom in on the area, the crypt pattern is different here. Of course, this does not fit with a xanthelasma or a scar from a healed gastric ulcer (GU) either for that matter. Interestingly, almost everyone thought that this was an EGC. However, THERE IS a distinct crypt pattern in the centre of the lesion. Furthermore, the lesion isn't red. Remember that cancers encourage the growth of small irregular capillaries which gives them a red colouration. Finally, it doesn't have the typical flat-elevated with a central depression (IIa+IIc) growth morphology. Therefore, your first guess should be a gastric adenoma! This is actually a gastric tubular adenoma which we found in an elderly frail patient with atrophic gastritis some 10 years ago. As she had some comorbidities and it was only harbouring LGD, we decided to keep an eye on the lesion on a yearly basis. The risk of progression is supposedly only 5% with tubular adenomas in the stomach. In contrast, villous adenomas are much more likely to progress (40%). The BSG gastric polyp guidelines have the references if you want to look this up. Of course, the issue is not entirely clear-cut as risk of progression also increases with the size of the lesion (and this is probably 2cm in size) and also with age (patient is now 86 yrs). In some ways, making an initial decision to either 'attack' or 'abort' would be easiest. After all, regular surveillance drains valuable resources and leaves you open to the possibility that at some point in the future, the patient is no longer a candidate for anything more invasive than a haircut but now the lesion under surveillance shows evidence of progression. Then your patient could well ask the legitimate question why you didn't go ahead when he was younger and fit enough but instead wasted his time with pointless surveillance?! My own preferred way to navigate this minefield is to openly discuss the three options with the patient;
I often wonder if its the personality type which dictates what patients prefer. Perhaps, those who think 'my glass is half empty' usually want to have their lesion resected immediately whilst people who regards their 'glass to be half full', prefer to hope for the best and get on with their lives? These three polyps were removed en-bloc from the proximal colon. In an earlier examination 2 SSL's had been removed. A subsequent colonoscopy finds no further polyps. WHAT WOULD YOU ADVISE AS REGARDS SURVEILLANCE?
■ There is no need for surveillance
You may be right if the patient is old and with multiple comorbidities!
■ Surveillance every 2 years
Yes, this is what guidelines usually recommend for 'SPS'
■ Surveillance every 5 years
Too long if pt fulfills WHO criteria for SPS
explanation
If course, this patient has 'Serrated Polyposis Syndrome'. It's important to recognise that the serrated polyp count is cumulative over multiple colonoscopies. The recently updated 2019 WHO criteria for Serrated Polyposis Syndrome recognize two types of the syndrome: a 'proximal phenotype' with serrated polyps proximal to the rectum, all being ≥5 mm in size, with at least two being ≥10 mm in size (criterion I 2019), and a more 'distal phenotype' with more than 20 serrated polyps of any size throughout the large bowel (criterion II 2019) [Gastroenterology 2020;158:1520–23]. Personally, I believe that there are more than two subtypes of the Serrated Polyposis Syndrome. There is accumulating evidence that the syndrome includes multiple conditions with variable phenotypes and with different risks of progression to CRC [Gut 2010;59:1094–1100]. This would explain the huge range of cancer risk (25%-70%) in published studies [GIE 2016;83:563–65]. Of course, the likely mixture of several 'syndromes', makes writing guidelines difficult. A recent consensus update by the US Multi-Society Task Force recommend offering a follow-up colonoscopy to average risk patients based on number and size of the SSL's found [GIE 2020;91:463–85]. Interestingly, the US guidelines make a distinction between 'hyperplastic polyps' and 'sessile serrated polyps' although pathologists can't reliably make that distinction. Furthermore, the guidelines excludes patients with an increased life-time risk of cancer which of course excludes patients with Serrated Polyposis Syndrome. I find it all somewhat confusing! James East's BSG guideline 2017 [ Gut 2017;0:1–16 ] recommend surveillance every other year whilst the more recent BSG/ACPGBI guideline of 2020 [ Gut 2020;69:201– 23 ] would seem to suggest 3 yr for all 'high risk cases. But these guidelines expressively don't cover pts with hereditary cancers. Hereditary cancers are instead covered by the BSG/ACPGBI 2019 guideline [ Gut 2019;0:1–34 ] which recommends annual surveillance until all polyps are cleared and then every 2 years. Finally, I admit that I also take the age of the patient into consideration as well as the presence of both serrated and adenomatous polyps. A 40 year old person is surely more likely to benefit from surveillance than a 75 year old person with multiple comorbidities? In particular, I would worry about a young patient, perhaps 35 year old, with 1-2 large serrated polyps and perhaps only a single adenoma. Current guidelines don't flag these individuals up but personally, I would organise another surveillance colonoscopy in a few years time. Clearly, more research is needed to unpick the different serrated sub-pathways ! This polyp was found in the transverse colon of a 60 year old man. WHAT IS THE LIKELY HISTOLOGY OF THE LESION?
■ Serrated polyp
Crypt openings look a little serrated but nothing else does!
■ Adenomatous polyp
This is not an adenomatous crypt pattern!
■ Hamartomatous polyp
When in doubt, it's hamartomatous !
■ Malignant polyp
The crypt pattern is too organised and regular for cancer!
explanation
Sadly, an intimate knowledge of the Kudo crypt patterns doesn't help you here! Adenomatous polyps should be covered with one of the following;
In this case, the closest match is of a serrated polyp, which have wide open crypts with a somewhat jagged outline. However, apart from the crypt openings, there is nothing on this which looks like a serrated polyp! If you can't see a crypt pattern, the lesion is likely to be malignant. Conversely, if the lesion does have crypts but still doesn't look familiar, its either a hamartomatous polyp or, perhaps less likely, a 'Traditional Serrated Adenoma'. This lesion turned out to be a hamartomatous polyp. Why a 60 year old man would grow a hamartomatous colonic polyp remains a mystery !
■ Inflammed granulation tissue
Was my first too! But it doesn't quite look right ...
■ Adenomatous polyp
Adenomatous polyps are usually smooth topped
■ Malignant polyp
An intramucosal cancer arising from the anastomosis
explanation
This patient had in the past undergone a Billroth II operation. You can see that the nodule is arising from the surgical anastomosis. Distal gastrectomy is a well-known risk factor for developing an anastomotic cancer later [Sitarz R. World J Gastro. 2012;18(25):3201–6]. The risk of anastomotic cancer steadily increases after surgery. About 15 years after surgery, the risk exceeds that of the background population (age- and sex-matched). For this reason, surveillance has been suggested to start 15 yrs after surgery. The case for surveillance was strengthened by the fact that dysplasia can often be found in random biopsies from the anastomosis several years before cancer develops. In spite of this surveillance in not recommended. A surveillance study in Amsterdam traced 500 patients who had undergone a distal gastrectomy for benign disease and only detected 10 cancers (6 were in an early stage). Furthermore, there was no survival advantage in the screened group after 10 years follow up [J Clin Pathol. 1984;37:748–54]. Other studies have also put the cancer yield by surveillance at around 2% and concluded that regular surveillance could not be recommended [Am J Surg 1977;134:581-4], [ Lancet 1977;ii467-9] [ Scan J Gastro 1981;suppl 16:169-71]. Nevertheless, my practise is to always take do 'opportunistic screening' by obtaining 6 biopsies or so from the gastric side of the anastomosis when I come across a case. On first glance the nodule appears to be inflamed granulation tissue only. However, there is an odd cleft in the centre and the base from which it arises is also nodular. The polyp was removed and was confirmed as harbouring intramucosal cancer. Of course, after finding dysplasia (which often does not progress) or IMca, these patients should be offered surveillance. |
Categories
All
|